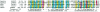Is the transportation highway the right road for hereditary spastic paraplegia? - PubMed (original) (raw)
Review
. 2002 Nov;71(5):1009-16.
doi: 10.1086/344206. Epub 2002 Sep 24.
- PMID: 12355399
- PMCID: PMC385081
- DOI: 10.1086/344206
Review
Is the transportation highway the right road for hereditary spastic paraplegia?
Andrew H Crosby et al. Am J Hum Genet. 2002 Nov.
Abstract
The term "hereditary spastic paraplegia" (HSP) refers to a genetically and clinically diverse group of disorders whose primary feature is progressive spasticity of the lower extremities. The condition arises because of degeneration of the longest motor and sensory axons on the spinal cord, which appear to be most sensitive to the underlying mutations. The marked genetic heterogeneity in HSP, with 20 loci chromosomally mapped and eight genes now identified, suggests that a number of defective cellular processes may be shown to result in the disease. Although previous studies have suggested a mitochondrial basis for at least one form of the disease, a mechanism common to a number of the other genes mutated in HSP has remained elusive until now. The identification of the most recent genes for the condition suggests that aberrant cellular-trafficking dynamics may be a common process responsible for the specific pattern of neurodegeneration seen in HSP.
Figures
Figure 1
Multiple sequence alignment of selected members of the ESP (MIT) domain–containing proteins. Sequences are indicated using their database accession number followed by the starting and the ending residues of the domain and by the species. The consensus present in ⩾70% of the sequences is given below the alignment; residues and colors are as follows: h (hydrophobic, blue), l (aliphatic, blue), K (lysine), p (polar, yellow), and R (arginine). Plus signs (+) indicate conserved, positively charged residues (lysine and arginine), which are colored in red in the alignment; minus signs (−) indicate conserved, negatively charged residues, which are indicated in pink. The secondary structure prediction (“Sec.Str.Pred.”) at the bottom of the alignment is derived from the alignment (H = helix predicted with expected average accuracy >82%; h = helix predicted with expected average accuracy <82%). Abbreviations: Hs, Homo sapiens; Mm, Mus musculus; and Sc, Saccharomyces cerevisiae.
Comment on
- A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10).
Reid E, Kloos M, Ashley-Koch A, Hughes L, Bevan S, Svenson IK, Graham FL, Gaskell PC, Dearlove A, Pericak-Vance MA, Rubinsztein DC, Marchuk DA. Reid E, et al. Am J Hum Genet. 2002 Nov;71(5):1189-94. doi: 10.1086/344210. Epub 2002 Sep 24. Am J Hum Genet. 2002. PMID: 12355402 Free PMC article.
Similar articles
- Hereditary spastic paraplegia: advances in genetic research. Hereditary Spastic Paraplegia Working group.
Fink JK, Heiman-Patterson T, Bird T, Cambi F, Dubé MP, Figlewicz DA, Fink JK, Haines JL, Heiman-Patterson T, Hentati A, Pericak-Vance MA, Raskind W, Rouleau GA, Siddique T. Fink JK, et al. Neurology. 1996 Jun;46(6):1507-14. doi: 10.1212/wnl.46.6.1507. Neurology. 1996. PMID: 8649538 Review. - Hereditary spastic paraplegia: genetic heterogeneity and genotype-phenotype correlation.
Fink JK, Hedera P. Fink JK, et al. Semin Neurol. 1999;19(3):301-9. doi: 10.1055/s-2008-1040846. Semin Neurol. 1999. PMID: 12194386 Review. - Advances in hereditary spastic paraplegia.
Fink JK. Fink JK. Curr Opin Neurol. 1997 Aug;10(4):313-8. doi: 10.1097/00019052-199708000-00006. Curr Opin Neurol. 1997. PMID: 9266155 Review. - [Molecular genetics study of hereditary spastic paraplegia accompanied by distal amyotrophy-an update].
Wang ZZ, Cen ZD, Luo W. Wang ZZ, et al. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2013 Aug;30(4):429-34. doi: 10.3760/cma.j.issn.1003-9406.2013.04.011. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2013. PMID: 23926010 Review. Chinese. - Early onset autosomal dominant spastic paraplegia caused by novel mutations in SPG3A.
Abel A, Fonknechten N, Hofer A, Dürr A, Cruaud C, Voit T, Weissenbach J, Brice A, Klimpe S, Auburger G, Hazan J. Abel A, et al. Neurogenetics. 2004 Dec;5(4):239-43. doi: 10.1007/s10048-004-0191-2. Epub 2004 Oct 28. Neurogenetics. 2004. PMID: 15517445
Cited by
- Monitoring Axonal Degeneration in Human Pluripotent Stem Cell Models of Hereditary Spastic Paraplegias.
Li XJ, Mou Y, Milton C, Chen Z. Li XJ, et al. Methods Mol Biol. 2022;2549:69-83. doi: 10.1007/7651_2021_379. Methods Mol Biol. 2022. PMID: 33772460 Free PMC article. - The Hsp60-(p.V98I) mutation associated with hereditary spastic paraplegia SPG13 compromises chaperonin function both in vitro and in vivo.
Bross P, Naundrup S, Hansen J, Nielsen MN, Christensen JH, Kruhøffer M, Palmfeldt J, Corydon TJ, Gregersen N, Ang D, Georgopoulos C, Nielsen KL. Bross P, et al. J Biol Chem. 2008 Jun 6;283(23):15694-700. doi: 10.1074/jbc.M800548200. Epub 2008 Apr 8. J Biol Chem. 2008. PMID: 18400758 Free PMC article. - Intragenic modifiers of hereditary spastic paraplegia due to spastin gene mutations.
Svenson IK, Kloos MT, Gaskell PC, Nance MA, Garbern JY, Hisanaga S, Pericak-Vance MA, Ashley-Koch AE, Marchuk DA. Svenson IK, et al. Neurogenetics. 2004 Sep;5(3):157-64. doi: 10.1007/s10048-004-0186-z. Epub 2004 Jul 10. Neurogenetics. 2004. PMID: 15248095 - A clinical, genetic and candidate gene study of Silver syndrome, a complicated form of hereditary spastic paraplegia.
Warner TT, Patel H, Proukakis C, Reed JA, McKie L, Wills A, Patton MA, Crosby AH. Warner TT, et al. J Neurol. 2004 Sep;251(9):1068-74. doi: 10.1007/s00415-004-0401-8. J Neurol. 2004. PMID: 15372247 - Loss of swiss cheese in Neurons Contributes to Neurodegeneration with Mitochondria Abnormalities, Reactive Oxygen Species Acceleration and Accumulation of Lipid Droplets in Drosophila Brain.
Melentev PA, Ryabova EV, Surina NV, Zhmujdina DR, Komissarov AE, Ivanova EA, Boltneva NP, Makhaeva GF, Sliusarenko MI, Yatsenko AS, Mohylyak II, Matiytsiv NP, Shcherbata HR, Sarantseva SV. Melentev PA, et al. Int J Mol Sci. 2021 Jul 31;22(15):8275. doi: 10.3390/ijms22158275. Int J Mol Sci. 2021. PMID: 34361042 Free PMC article.
References
Electronic-Database Information
- HUGO Gene Nomenclature Committee, http://www.gene.ucl.ac.uk/nomenclature/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for SPG1 [MIM <312900>], SPG2 [MIM <312920>], SPG3A [MIM <182600>], SPG4 [MIM <182601>], SPG5A [MIM <270800>], SPG6 [MIM <600363>], SPG7 [MIM <600146>], SPG8 [MIM <603563>], SPG9 [MIM <601162>], SPG10 [MIM <604187>], SPG11 [MIM <604360>], SPG12 [MIM <604805>], SPG13 [MIM <605280>], SPG14 [MIM <605229>], SPG15 [MIM <606859>], SPG16 [MIM <300266>], SPG17 [MIM <270685>], SPG19 [MIM <607152>], SPG20 [MIM <275900>], autosomal recessive spastic ataxia of Charlevoix-Seguenay [MIM <270550>], Charcot-MarieTooth type 2A [MIM <118210>], amyotrophic lateral sclerosis 2 [MIM <205100>], CHAC [MIM <200150>], and NPC1 [MIM <257220>])
References
- Apodaca G (2001) Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic 2:149–159 - PubMed
- Almenar-Queralt A, Goldstein LS (2001) Linkers, packages and pathways: new concepts in axonal transport. Curr Opin Neurobiol 11:550–557 - PubMed
- Barr VA, Phillips SA, Taylor SI, Haft CR (2000) Overexpression of a novel sorting nexin, SNX15, affects endosome morphology and protein trafficking. Traffic 1:904–916 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources