Molecular correlates of the M-current in cultured rat hippocampal neurons - PubMed (original) (raw)
Molecular correlates of the M-current in cultured rat hippocampal neurons
M M Shah et al. J Physiol. 2002.
Abstract
M-type K(+) currents (I(K(M))) play a key role in regulating neuronal excitability. In sympathetic neurons, M-channels are thought to be composed of a heteromeric assembly of KCNQ2 and KCNQ3 K(+) channel subunits. Here, we have tried to identify the KCNQ subunits that are involved in the generation of I(K(M)) in hippocampal pyramidal neurons cultured from 5- to 7-day-old rats. RT-PCR of either CA1 or CA3 regions revealed the presence of KCNQ2, KCNQ3, KCNQ4 and KCNQ5 subunits. Single-cell PCR of dissociated hippocampal pyramidal neurons gave detectable signals for only KCNQ2, KCNQ3 and KCNQ5; where tested, most also expressed mRNA for the vesicular glutamate transporter VGLUT1. Staining for KCNQ2 and KCNQ5 protein showed punctate fluorescence on both the somata and dendrites of hippocampal neurons. Staining for KCNQ3 was diffusely distributed whereas KCNQ4 was undetectable. In perforated patch recordings, linopirdine, a specific M-channel blocker, fully inhibited I(K(M)) with an IC(50) of 3.6 +/- 1.5 microM. In 70 % of these cells, TEA fully suppressed I(K(M)) with an IC(50) of 0.7 +/- 0.1 mM. In the remaining cells, TEA maximally reduced I(K(M)) by only 59.7 +/- 5.2 % with an IC(50) of 1.4 +/- 0.3 mM; residual I(K(M)) was abolished by linopirdine. Our data suggest that KCNQ2, KCNQ3 and KCNQ5 subunits contribute to I(K(M)) in these neurons and that the variations in TEA sensitivity may reflect differential expression of KCNQ2, KCNQ3 and KCNQ5 subunits.
Figures
Figure 1. Reverse-transcription PCR analysis from rat pyramidal hippocampal neurons
A, RT-PCR was performed using 0.2 μg of DNase I-treated, total RNA from CA1 or CA3 hippocampal regions isolated from two different 5- to 7-day-old rats. See Methods for intron-spanning primer pairs. Control reactions were performed using plasmids containing cDNA sequences encoding rKCNQ2, rKCNQ3, rKCNQ4 and hKCNQ5 (labelled 2 to 5). -ve, absence of template; M, 1 kb plus ladder (Life Technologies). B, representative single-cell PCRs for KCNQ2-5 and VGLUT1 mRNAs obtained from three single hippocampal neurons (labelled A, B and C). Cells were isolated from the CA1 and CA3 regions of 7-day-old rats and subsequently cultured in vitro for 10 days. Primer pairs used are described in Methods. Control reactions were performed using plasmids containing cDNA sequences encoding rKCNQ2, rKCNQ3, rKCNQ4 and hKCNQ5 (labelled 2 to 5). Hipp, rat hippocampus cDNA; -ve, absence of template; M, 1 kb plus ladder.
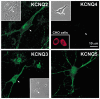
Figure 2. Immunodetection of KCNQ channel subunits in hippocampal neurons
Confocal images (10 stacked at 0.5 μm intervals) of KCNQ2, KCNQ3, KCNQ4 and KCNQ5 immunostaining in hippocampal pyramidal neurons cultured in vitro for 10 days. Note the plasma membrane staining for KCNQ2 whereas KCNQ3 and KCNQ5 antibodies appear to label both the cell surface and intracellular components. Inset, staining of CHO cells expressing exogenous KCNQ4 subunits with the anti-KCNQ4 antibody. Scale bar, 10 μm. The greyscale insets show phase-contrast images of the same fields at 40 % magnification.
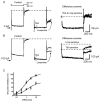
Figure 3. Pharmacological characterization of _I_K(M) in hippocampal pyramidal cells
A and B show currents recorded from two cultured neurons in the absence (Control) and presence of oxotremorine-M (Oxo-M) and linopirdine, respectively. Cells were held at −20 mV and currents evoked by stepping to −50 mV (voltage protocol shown in A). The difference currents were obtained by subtracting the control trace at −50 mV from that in the presence of the respective compounds. The horizontal dashed lines mark the initial baseline holding current. The scale bars shown in B also apply to A. C, average concentration-inhibition curve for linopirdine fitted using eqn (1). Each data point is the mean ±
s.e.m
. of 3–13 observations. (The slow component of the difference current in A may reflect the hyperpolarization-activated cation current _I_Q/_I_h, which is enhanced by oxotremorine-M: see Colino & Halliwell, 1993.)
Figure 4. Effects of TEA on _I_K(M) recorded in hippocampal pyramidal neurons
A and B show representative examples of currents recorded from two different cells with differential sensitivity to 10 m
m
TEA. Superimposed are currents in the presence of TEA and co-applied TEA and linopirdine (Linop, 30 μM). The currents were recorded by applying the voltage step shown in A. C, cumulative concentration-inhibition curves for TEA. Squares and circles represent curves for cells in which TEA abolished _I_K(M) (_n_= 7) and cells in which TEA only partially inhibited _I_K(M) (_n_= 3), respectively.
Similar articles
- Stoichiometry of expressed KCNQ2/KCNQ3 potassium channels and subunit composition of native ganglionic M channels deduced from block by tetraethylammonium.
Hadley JK, Passmore GM, Tatulian L, Al-Qatari M, Ye F, Wickenden AD, Brown DA. Hadley JK, et al. J Neurosci. 2003 Jun 15;23(12):5012-9. doi: 10.1523/JNEUROSCI.23-12-05012.2003. J Neurosci. 2003. PMID: 12832524 Free PMC article. - Single-channel analysis of KCNQ K+ channels reveals the mechanism of augmentation by a cysteine-modifying reagent.
Li Y, Gamper N, Shapiro MS. Li Y, et al. J Neurosci. 2004 Jun 2;24(22):5079-90. doi: 10.1523/JNEUROSCI.0882-04.2004. J Neurosci. 2004. PMID: 15175377 Free PMC article. - Effects of KCNQ channel modulators on the M-type potassium current in primate retinal pigment epithelium.
Pattnaik BR, Hughes BA. Pattnaik BR, et al. Am J Physiol Cell Physiol. 2012 Mar 1;302(5):C821-33. doi: 10.1152/ajpcell.00269.2011. Epub 2011 Nov 30. Am J Physiol Cell Physiol. 2012. PMID: 22135213 Free PMC article. - KCNQ potassium channels: physiology, pathophysiology, and pharmacology.
Robbins J. Robbins J. Pharmacol Ther. 2001 Apr;90(1):1-19. doi: 10.1016/s0163-7258(01)00116-4. Pharmacol Ther. 2001. PMID: 11448722 Review. - M-channels: neurological diseases, neuromodulation, and drug development.
Cooper EC, Jan LY. Cooper EC, et al. Arch Neurol. 2003 Apr;60(4):496-500. doi: 10.1001/archneur.60.4.496. Arch Neurol. 2003. PMID: 12707061 Review.
Cited by
- Impaired Kv7 channel function in cerebral arteries of a tauopathy mouse model (rTg4510).
de Jong IEM, Jepps TA. de Jong IEM, et al. Physiol Rep. 2018 Dec;6(23):e13920. doi: 10.14814/phy2.13920. Physiol Rep. 2018. PMID: 30548427 Free PMC article. - Participation of calcium-permeable AMPA receptors in the regulation of epileptiform activity of hippocampal neurons.
Zinchenko VP, Teplov IY, Kosenkov AM, Gaidin SG, Kairat BK, Tuleukhanov ST. Zinchenko VP, et al. Front Synaptic Neurosci. 2024 Mar 20;16:1349984. doi: 10.3389/fnsyn.2024.1349984. eCollection 2024. Front Synaptic Neurosci. 2024. PMID: 38577639 Free PMC article. - Novel role of KCNQ2/3 channels in regulating neuronal cell viability.
Zhou X, Wei J, Song M, Francis K, Yu SP. Zhou X, et al. Cell Death Differ. 2011 Mar;18(3):493-505. doi: 10.1038/cdd.2010.120. Epub 2010 Oct 1. Cell Death Differ. 2011. PMID: 20885443 Free PMC article. - Contributions of Kv7-mediated potassium current to sub- and suprathreshold responses of rat layer II/III neocortical pyramidal neurons.
Guan D, Higgs MH, Horton LR, Spain WJ, Foehring RC. Guan D, et al. J Neurophysiol. 2011 Oct;106(4):1722-33. doi: 10.1152/jn.00211.2011. Epub 2011 Jun 22. J Neurophysiol. 2011. PMID: 21697446 Free PMC article. - Sub- and suprathreshold adaptation currents have opposite effects on frequency tuning.
Deemyad T, Kroeger J, Chacron MJ. Deemyad T, et al. J Physiol. 2012 Oct 1;590(19):4839-58. doi: 10.1113/jphysiol.2012.234401. Epub 2012 Jun 25. J Physiol. 2012. PMID: 22733663 Free PMC article.
References
- Brown DA. M currents. In: Narahashi T, editor. Ion Channels. Vol. 1. New York: Plenum Press; 1988. pp. 55–99. - PubMed
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neuron. Nature. 1980;283:673–676. - PubMed
- Colino A, Halliwell JV. Carbachol potentiates Q current and activates a calcium-dependent non-specific conductance in rat hippocampus in vitro. European Journal of Neuroscience. 1993;5:1198–1209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous