Human Mcm proteins at a replication origin during the G1 to S phase transition - PubMed (original) (raw)
Human Mcm proteins at a replication origin during the G1 to S phase transition
Daniel Schaarschmidt et al. Nucleic Acids Res. 2002.
Abstract
Previous work with yeast cells and with Xenopus egg extracts had shown that eukaryotic pre-replication complexes assemble on chromatin in a step-wise manner whereby specific loading factors promote the recruitment of essential Mcm proteins at pre-bound origin recognition complexes (ORC with proteins Orc1p-Orc6p). While the order of assembly--Mcm binding follows ORC binding--seems to be conserved in cycling mammalian cells in culture, it has not been determined whether mammalian Mcm proteins associate with ORC-bearing chromatin sites. We have used a chromatin immunoprecipitation approach to investigate the site of Mcm binding in a genomic region that has previously been shown to contain an ORC-binding site and an origin of replication. Using chromatin from HeLa cells in G1 phase, antibodies against Orc2p as well as antibodies against Mcm proteins specifically immunoprecipitate chromatin enriched for a DNA region that includes a replication origin. However, with chromatin from cells in S phase, only Orc2p-specific antibodies immunoprecipitate the origin-containing DNA region while Mcm-specific antibodies immunoprecipitate chromatin with DNA from all parts of the genomic region investigated. Thus, human Mcm proteins first assemble at or adjacent to bound ORC and move to other sites during genome replication.
Figures
Figure 1
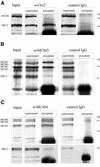
Orc2p and Mcm proteins on crosslinked chromatin. (A) Immunoprecipitations with antibodies against Orc2p (α-Orc2) and with non-specific control antibodies (IgG). Input (crosslinked, nuclease-treated chromatin before immunoprecipitation), supernatants (remaining chromatin after treatment with antibodies and protein A–Sepharose) and immunoprecipitates were analyzed in western blots with Mcm3p-specific (MCM3) and with Orc2p-specific antibodies (ORC2). (B) Immunoprecipitations with antibodies against Mcm3p (α-MCM3) and with non-specific control antibodies (IgG). The input, supernatant and precipitated samples were western blotted and analyzed with antibodies against Mcm3p (MCM3), Mcm4p (MCM4), Mcm5p (MCM5) and Mcm7p (MCM7) as well as with antibodies against Orc2p (ORC2). (C) Immunoprecipitation with antibodies against Mcm4p (α-MCM4) and with non-specific control antibodies (IgG). The input, supernatant and precipitated samples were western blotted and analyzed with Mcm4p, Mcm6p and Mcm7p antibodies as well as with antibodies against Orc2p as indicated.
Figure 2
ChIP assay on crosslinked chromatin from synchronized HeLa cells. Crosslinked chromatin from G1 phase cells and S phase cells was immunoprecipitated with antibodies against Mcm3p (α-MCM3). The input, supernatant and precipitated samples were western blotted and analyzed with Mcm3p, Mcm5p and Orc2p antibodies as well as with antibodies against the large subunit of RPA (RPA70) as indicated. Synchronization was verified by analysis of DNA content on a flow cytometer (shown in the conventional manner).
Figure 3
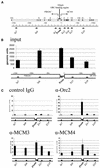
(Opposite) Specific DNA regions in immunoprecipitated crosslinked chromatin from unsynchronized HeLa cells. (A) Genomic organization of the analyzed region encompassing human genes PRKDC and MCM4 (70). In the double line diagram, exons are shown as black boxes and arrows indicate the starts and directions of transcription. In the single line diagram, divergent arrowheads show regions complementary to the PCR primers used. (B) Input. DNA was extracted from crosslinked chromatin before immunoprecipitation and amplified by quantitative PCR using the primer sets indicated. The results are expressed in ‘genomic units’ relative to serially diluted genomic control DNA. (C) Immunoprecipitated chromatin. Quantitative real-time PCR with DNA templates extracted from chromatin precipitated with control antibodies and with antibodies against Orc2p, Mcm3p and Mcm4p as indicated.
Figure 4
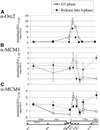
Specific DNA regions in immunoprecipitated crosslinked chromatin from cells in G1 phase and S phase. HeLa cells were synchronized in G1 phase (left) or released into S phase for 4 h (right) and were subjected to ChIP analysis. Quantitative real-time PCR was performed with DNA templates extracted from chromatin precipitated with antibodies against (A) Orc2p, (B) Mcm3p and (C) Mcm4p. We show the results of three independent experiments. (Below) Schematic representation of the genomic region analyzed with the PCR-amplified segments indicated by arrowheads.
Figure 5
Enrichment of specific DNA sequences in immunoprecipitates. Quantitative PCR gives the results in ‘genomic units’ relative to serially diluted purified genomic DNA. The ratios of precipitated over input genomic units are multiplied by 100 and plotted against the primer sites on the analyzed region. The results of the precipitations with Orc2 antibodies are summarized in (A), with Mcm3 antibodies in (B) and with Mcm4 antibodies in (C). Open squares indicate the mean enrichment of the G1 phase samples and closed circles indicate the mean enrichment of the samples from S phase cells.
Similar articles
- The Xenopus origin recognition complex is essential for DNA replication and MCM binding to chromatin.
Romanowski P, Madine MA, Rowles A, Blow JJ, Laskey RA. Romanowski P, et al. Curr Biol. 1996 Nov 1;6(11):1416-25. doi: 10.1016/s0960-9822(96)00746-4. Curr Biol. 1996. PMID: 8939603 - MCM2-7 complexes bind chromatin in a distributed pattern surrounding the origin recognition complex in Xenopus egg extracts.
Edwards MC, Tutter AV, Cvetic C, Gilbert CH, Prokhorova TA, Walter JC. Edwards MC, et al. J Biol Chem. 2002 Sep 6;277(36):33049-57. doi: 10.1074/jbc.M204438200. Epub 2002 Jun 26. J Biol Chem. 2002. PMID: 12087101 - Site-specific loading of an MCM protein complex in a DNA replication initiation zone upstream of the c-MYC gene in the HeLa cell cycle.
Kinoshita Y, Johnson EM. Kinoshita Y, et al. J Biol Chem. 2004 Aug 20;279(34):35879-89. doi: 10.1074/jbc.M401640200. Epub 2004 Jun 9. J Biol Chem. 2004. PMID: 15190069 - The MCM complex: its role in DNA replication and implications for cancer therapy.
Lei M. Lei M. Curr Cancer Drug Targets. 2005 Aug;5(5):365-80. doi: 10.2174/1568009054629654. Curr Cancer Drug Targets. 2005. PMID: 16101384 Review. - The 'ORC cycle': a novel pathway for regulating eukaryotic DNA replication.
DePamphilis ML. DePamphilis ML. Gene. 2003 May 22;310:1-15. doi: 10.1016/s0378-1119(03)00546-8. Gene. 2003. PMID: 12801628 Review.
Cited by
- Segregation of replicative DNA polymerases during S phase: DNA polymerase ε, but not DNA polymerases α/δ, are associated with lamins throughout S phase in human cells.
Vaara M, Itkonen H, Hillukkala T, Liu Z, Nasheuer HP, Schaarschmidt D, Pospiech H, Syväoja JE. Vaara M, et al. J Biol Chem. 2012 Sep 28;287(40):33327-38. doi: 10.1074/jbc.M112.357996. Epub 2012 Aug 10. J Biol Chem. 2012. PMID: 22887995 Free PMC article. - Site-specific interaction of the murine pre-replicative complex with origin DNA: assembly and disassembly during cell cycle transit and differentiation.
Zellner E, Herrmann T, Schulz C, Grummt F. Zellner E, et al. Nucleic Acids Res. 2007;35(20):6701-13. doi: 10.1093/nar/gkm555. Epub 2007 Oct 4. Nucleic Acids Res. 2007. PMID: 17916579 Free PMC article. - Identification of new human origins of DNA replication by an origin-trapping assay.
Gerhardt J, Jafar S, Spindler MP, Ott E, Schepers A. Gerhardt J, et al. Mol Cell Biol. 2006 Oct;26(20):7731-46. doi: 10.1128/MCB.01392-06. Epub 2006 Sep 5. Mol Cell Biol. 2006. PMID: 16954389 Free PMC article. - Chromatin immunoprecipitation to investigate origin association of replication factors in mammalian cells.
Leman AR, Noguchi E. Leman AR, et al. Methods Mol Biol. 2014;1170:539-47. doi: 10.1007/978-1-4939-0888-2_30. Methods Mol Biol. 2014. PMID: 24906335 Free PMC article. - Identification and characterization of a novel component of the human minichromosome maintenance complex.
Sakwe AM, Nguyen T, Athanasopoulos V, Shire K, Frappier L. Sakwe AM, et al. Mol Cell Biol. 2007 Apr;27(8):3044-55. doi: 10.1128/MCB.02384-06. Epub 2007 Feb 12. Mol Cell Biol. 2007. PMID: 17296731 Free PMC article.
References
- Kearsey S.E. and Labib,K. (1998) MCM proteins: evolution, properties and role in DNA replication. Biochim. Biophys. Acta, 1398, 113–136. - PubMed
- Lei M. and Tye,B.K. (2001) Initiating DNA synthesis: from recruiting to activating the MCM complex. J. Cell Sci., 114, 1447–1454. - PubMed
- Pasion S.G. and Forsburg,S.L. (2001) Deconstructing a conserved protein family: the role of MCM proteins in eukaryotic DNA replication. Genet. Eng., 23, 129–155. - PubMed
- Tye B.K. (1999) MCM proteins in DNA replication. Annu. Rev. Biochem., 68, 649–686. - PubMed
- Aparicio O.M., Weinstein,D.M. and Bell,S.P. (1997) Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell, 91, 59–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases