Biochemical analysis of tau proteins in argyrophilic grain disease, Alzheimer's disease, and Pick's disease : a comparative study - PubMed (original) (raw)
Comparative Study
Biochemical analysis of tau proteins in argyrophilic grain disease, Alzheimer's disease, and Pick's disease : a comparative study
Victoria Zhukareva et al. Am J Pathol. 2002 Oct.
Abstract
Although argyrophilic grain disease is characterized histopathologically by tau-positive lesions known as argyrophilic grains located predominantly in limbic brain regions in the absence of other diagnostic neuropathologies, the biochemical correlates of argyrophilic grains in gray and white matter have not been reported. Thus, we analyzed insoluble (pathological) tau proteins in five argyrophilic grain disease brains in comparison with those seen in Alzheimer's disease and Pick's disease. Analyses of separately dissected gray and white matter samples from various cortical regions revealed that pathological tau in argyrophilic grain disease was confined primarily to mediotemporal neocortical gray and adjacent white matter, and also to the allocortex, amygdala, and hippocampus. The amounts of sarcosyl-insoluble tau in all five cases were substantially lower than in Alzheimer's disease and Pick's disease, but the amounts of sarcosyl-insoluble tau in white matter were higher or comparable to that detected in gray matter from the same region, which distinguishes argyrophilic grain disease from Alzheimer's disease. The banding patterns of tau isoforms in argyrophilic grain disease varied: in three cases they were similar to Alzheimer's disease, but in two other cases, 4 microtubule binding repeat (4R) tau predominated, which distinguishes argyrophilic grain disease from classical Pick's disease. The differences between these three diseases were re-enforced by the predominance of straight tau filaments from argyrophilic grain disease brains. Thus, we conclude that argyrophilic grain disease is a distinct tauopathy characterized by prominent accumulation of argyrophilic grains in limbic brain regions in association with the characteristic tau biochemical and ultrastructural profile reported here.
Figures
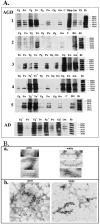
Figure 1.
Neuropathology of AGD cases. Sections of hippocampus, amygdala, entorhinal cortex, and temporal cortex of different AGD cases were stained with τ mAb PHF1 (1:3000). AGs are seen in amygdala (A, case 1, arrows show the punctate AGs distribution; B, case 5; C, case 2), hippocampus (D, case 1; F, case 3) and temporal cortex (C, case 4), whereas AD-type pathology and AGs are seen in entorhinal cortex (E, case 3). E: Glial inclusions are seen in temporal cortex (case 2). Original magnifications: ×2300 (A, C–F); ×7750 (B).
Figure 2.
Biochemical and ultrastructural analysis of sarcosyl-insoluble τ. A: Dephosphorylated samples from different brain regions were resolved onto 7.5% SDS-polyacrylamide gel. Twenty percent of total sarcosyl-insoluble material from each sample was loaded in each lane, and nitrocellulose replicas were probed with a mixture of mAbs Tau14 and Tau46. A: Brain region-specific distribution of pathological τ. F, frontal; T, temporal; P, parietal; O, occipital cortex; C, cerebellum; Hipp, hippocampus; Amy, amygdala; Th, thalamus; E, entorhinal cortex; BG, basal ganglia; g, gray matter; w, white matter. A mixture of six recombinant human τ isoforms is shown on the right for the comparison (Rt). Case numbers are shown on the left and on the right. Asterisks indicate samples with 30% reduction of loaded material to avoid the saturation. B: Dispersed τ filaments were isolated from frozen temporal cortical gray and white matter (case 2) adjacent to tissue used for biochemical analysis. a: Electron microphotographs of τ filament stained with 0.4% uranyl acetate. Microphotographs were taken at a nominal original magnification of ×120,000. b: Immunogold labeling of τ filaments using PHF1 antibodies.
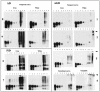
Figure 3.
Sequential extraction of τ proteins from AGD, AD, and PiD cases: Western blot analysis. Temporal cortex (AD, cases 8 and 9; PiD, cases 6 and 7; AGD, cases 1, 2, and 5) and entorhinal cortex and amygdala (AGD, case 4) with abundant τ pathology were studied after repeated extraction with buffers of increasing stringency as described in Materials and Methods. Lane 1: Buffer A (first extraction is shown); lane 2: 1% Triton X-100 in buffer A; lane 3: RIPA buffer; lane 4: 2% SDS; lane 5: 70% formic acid. A mixture of mAbs Tau14 and Tau46 were used for the Western blot analysis. An equal amount of material from each extraction step was loaded. Highly insoluble aggregated τ species were detected in AGD cases 2, 4, and 5 (arrowheads).
Similar articles
- Sporadic Pick's disease: a tauopathy characterized by a spectrum of pathological tau isoforms in gray and white matter.
Zhukareva V, Mann D, Pickering-Brown S, Uryu K, Shuck T, Shah K, Grossman M, Miller BL, Hulette CM, Feinstein SC, Trojanowski JQ, Lee VM. Zhukareva V, et al. Ann Neurol. 2002 Jun;51(6):730-9. doi: 10.1002/ana.10222. Ann Neurol. 2002. PMID: 12112079 - Novel tau filament fold in corticobasal degeneration.
Zhang W, Tarutani A, Newell KL, Murzin AG, Matsubara T, Falcon B, Vidal R, Garringer HJ, Shi Y, Ikeuchi T, Murayama S, Ghetti B, Hasegawa M, Goedert M, Scheres SHW. Zhang W, et al. Nature. 2020 Apr;580(7802):283-287. doi: 10.1038/s41586-020-2043-0. Epub 2020 Feb 12. Nature. 2020. PMID: 32050258 Free PMC article. - Development of a novel tau propagation mouse model endogenously expressing 3 and 4 repeat tau isoforms.
Hosokawa M, Masuda-Suzukake M, Shitara H, Shimozawa A, Suzuki G, Kondo H, Nonaka T, Campbell W, Arai T, Hasegawa M. Hosokawa M, et al. Brain. 2022 Mar 29;145(1):349-361. doi: 10.1093/brain/awab289. Brain. 2022. PMID: 34515757 - [Neuropathology of tauopathy].
Yoshida M. Yoshida M. Brain Nerve. 2013 Dec;65(12):1445-58. Brain Nerve. 2013. PMID: 24323931 Review. Japanese. - Ordered Assembly of Tau Protein and Neurodegeneration.
Goedert M, Spillantini MG. Goedert M, et al. Adv Exp Med Biol. 2019;1184:3-21. doi: 10.1007/978-981-32-9358-8_1. Adv Exp Med Biol. 2019. PMID: 32096024 Review.
Cited by
- A quantitative study of α-synuclein pathology in fifteen cases of dementia associated with Parkinson disease.
Armstrong RA, Kotzbauer PT, Perlmutter JS, Campbell MC, Hurth KM, Schmidt RE, Cairns NJ. Armstrong RA, et al. J Neural Transm (Vienna). 2014 Feb;121(2):171-81. doi: 10.1007/s00702-013-1084-z. Epub 2013 Aug 31. J Neural Transm (Vienna). 2014. PMID: 23996276 Free PMC article. - Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine.
Irwin DJ, Cairns NJ, Grossman M, McMillan CT, Lee EB, Van Deerlin VM, Lee VM, Trojanowski JQ. Irwin DJ, et al. Acta Neuropathol. 2015 Apr;129(4):469-91. doi: 10.1007/s00401-014-1380-1. Epub 2014 Dec 31. Acta Neuropathol. 2015. PMID: 25549971 Free PMC article. - Primary retinal tauopathy: A tauopathy with a distinct molecular pattern.
Walkiewicz G, Ronisz A, Van Ginderdeuren R, Lemmens S, Bouwman FH, Hoozemans JJM, Morrema THJ, Rozemuller AJ, Hart de Ruyter FJ, De Groef L, Stalmans I, Thal DR. Walkiewicz G, et al. Alzheimers Dement. 2024 Jan;20(1):330-340. doi: 10.1002/alz.13424. Epub 2023 Aug 24. Alzheimers Dement. 2024. PMID: 37615275 Free PMC article. - Tau protein is cross-linked by transglutaminase in P301L tau transgenic mice.
Halverson RA, Lewis J, Frausto S, Hutton M, Muma NA. Halverson RA, et al. J Neurosci. 2005 Feb 2;25(5):1226-33. doi: 10.1523/JNEUROSCI.3263-04.2005. J Neurosci. 2005. PMID: 15689560 Free PMC article. - A quantitative study of tau pathology in 11 cases of chronic traumatic encephalopathy.
Armstrong RA, McKee AC, Stein TD, Alvarez VE, Cairns NJ. Armstrong RA, et al. Neuropathol Appl Neurobiol. 2017 Feb;43(2):154-166. doi: 10.1111/nan.12323. Epub 2016 Apr 15. Neuropathol Appl Neurobiol. 2017. PMID: 26998921 Free PMC article.
References
- Tolnay M, Spillantini MG, Goedert M, Ulrich J, Langui D, Probst A: Argyrophilic grain disease: widespread hypophosphorylation of tau protein in limbic neurons. Acta Neuropathol 1997, 93:477-484 - PubMed
- Braak H, Braak E: Argyrophilic grain disease: frequency of occurrence in different age categories and neuropathological diagnostic criteria. J Neural Transm 1998, 105:801-819 - PubMed
- Tolnay M, Probst A: Review: tau protein pathology in Alzheimer’s disease and related disorders. Neuropathol Appl Neurobiol 1999, 25:171-187 - PubMed
- Braak H, Del Tredici K, Bohl J, Bratzke H, Braak E: Pathological changes in the parahippocampal region in select non-Alzheimer’s dementias. Ann NY Acad Sci 2000, 911:221-239 - PubMed
- Braak H, Braak E: Cortical and subcortical argyrophilic grains characterize a disease associated with adult onset dementia. Neuropathol Appl Neurobiol 1989, 15:13-26 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases