A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation - PubMed (original) (raw)
. 2002 Oct 7;196(7):887-96.
doi: 10.1084/jem.20012044.
Korbinian Brand, Sabine Grüner, Sharon Page, Elke Müller, Iris Müller, Wolfgang Bergmeier, Thomas Richter, Michael Lorenz, Ildiko Konrad, Bernhard Nieswandt, Meinrad Gawaz
Affiliations
- PMID: 12370251
- PMCID: PMC2194025
- DOI: 10.1084/jem.20012044
A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation
Steffen Massberg et al. J Exp Med. 2002.
Abstract
The contribution of platelets to the process of atherosclerosis remains unclear. Here, we show in vivo that platelets adhere to the vascular endothelium of the carotid artery in ApoE(-)(/)(-) mice before the development of manifest atherosclerotic lesions. Platelet-endothelial cell interaction involved both platelet glycoprotein (GP)Ibalpha and GPIIb-IIIa. Platelet adhesion to the endothelium coincides with inflammatory gene expression and preceded atherosclerotic plaque invasion by leukocytes. Prolonged blockade of platelet adhesion in ApoE(-)(/)(-) mice profoundly reduced leukocyte accumulation in the arterial intima and attenuated atherosclerotic lesion formation in the carotid artery bifurcation, the aortic sinus, and the coronary arteries. These findings establish the platelet as a major player in initiation of the atherogenetic process.
Figures
Figure 1.
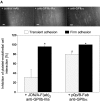
Platelet adhesion to the endothelium of the common carotid artery in ApoE −/− mice in vivo. (A) Assessment of platelet adhesion at the carotid bifurcation (lesion-prone-site, A) and within the proximal portion of the common carotid artery. For in vivo microscopy, two imaginary perpendicular axes were dropped through the origin of the internal and the external common carotid artery. Platelet and leukocyte adhesion were determined at high magnification (500-fold) in a 200 × 100 μm area adjacent to a third line, connecting the perpendicular axes at their intersection with the vessel wall (lesion-prone site, A). In a subset of experiments, platelet adhesion was also determined in the proximal portion of the common carotid artery 500 μm upstream of the carotid bifurcation (nonlesion-prone site, B). ACC, common carotid artery; ACE, external carotid artery; ACI, internal carotid artery. (B) Platelet–endothelial cell interactions were investigated in 6-, 8-, 10-, 12-, 16-, and 22-wk-old ApoE −/− mice by in vivo fluorescence microscopy of the common carotid artery in situ. Wild-type animals served as controls. The top and bottom panels summarize transient and firm platelet adhesion, respectively, of 10 experiments per group. Platelets were classified according to their interaction with the endothelial cell lining as described previously (reference 5) and are given per mm2 of vessel surface. (C) The microphotographs show representative in vivo fluorescence microscopy images at distinct time points. White arrows indicate adherent platelets, and the black arrow indicates the area of a fatty streak. The small inserts demonstrate histological sections of the corresponding carotid artery under investigation (original magnification: 200-fold). Underneath, the in vivo microscopic images representative en face carotid arteries, stained with Sudan III, are given. (D) Assessment of platelet adhesion to lesion-prone (white bars) and nonlesion-prone sites (black bars). Platelet adhesion was quantified in the proximal (nonlesion-prone) carotid artery and adjacent to the carotid bifurcation (lesion-prone) in both, young (10-wk-old) and old (22-wk-old) ApoE −/− mice (left). The photomicrograph in the middle shows a representative carotid artery, stained with Sudan III. Atherosclerotic plaque formation occurs preferentially at the carotid bifurcation. Representative histological sections of the carotid bifurcation and the proximal common carotid artery are presented in the right panels (original magnification: 200-fold).
Figure 1.
Platelet adhesion to the endothelium of the common carotid artery in ApoE −/− mice in vivo. (A) Assessment of platelet adhesion at the carotid bifurcation (lesion-prone-site, A) and within the proximal portion of the common carotid artery. For in vivo microscopy, two imaginary perpendicular axes were dropped through the origin of the internal and the external common carotid artery. Platelet and leukocyte adhesion were determined at high magnification (500-fold) in a 200 × 100 μm area adjacent to a third line, connecting the perpendicular axes at their intersection with the vessel wall (lesion-prone site, A). In a subset of experiments, platelet adhesion was also determined in the proximal portion of the common carotid artery 500 μm upstream of the carotid bifurcation (nonlesion-prone site, B). ACC, common carotid artery; ACE, external carotid artery; ACI, internal carotid artery. (B) Platelet–endothelial cell interactions were investigated in 6-, 8-, 10-, 12-, 16-, and 22-wk-old ApoE −/− mice by in vivo fluorescence microscopy of the common carotid artery in situ. Wild-type animals served as controls. The top and bottom panels summarize transient and firm platelet adhesion, respectively, of 10 experiments per group. Platelets were classified according to their interaction with the endothelial cell lining as described previously (reference 5) and are given per mm2 of vessel surface. (C) The microphotographs show representative in vivo fluorescence microscopy images at distinct time points. White arrows indicate adherent platelets, and the black arrow indicates the area of a fatty streak. The small inserts demonstrate histological sections of the corresponding carotid artery under investigation (original magnification: 200-fold). Underneath, the in vivo microscopic images representative en face carotid arteries, stained with Sudan III, are given. (D) Assessment of platelet adhesion to lesion-prone (white bars) and nonlesion-prone sites (black bars). Platelet adhesion was quantified in the proximal (nonlesion-prone) carotid artery and adjacent to the carotid bifurcation (lesion-prone) in both, young (10-wk-old) and old (22-wk-old) ApoE −/− mice (left). The photomicrograph in the middle shows a representative carotid artery, stained with Sudan III. Atherosclerotic plaque formation occurs preferentially at the carotid bifurcation. Representative histological sections of the carotid bifurcation and the proximal common carotid artery are presented in the right panels (original magnification: 200-fold).
Figure 1.
Platelet adhesion to the endothelium of the common carotid artery in ApoE −/− mice in vivo. (A) Assessment of platelet adhesion at the carotid bifurcation (lesion-prone-site, A) and within the proximal portion of the common carotid artery. For in vivo microscopy, two imaginary perpendicular axes were dropped through the origin of the internal and the external common carotid artery. Platelet and leukocyte adhesion were determined at high magnification (500-fold) in a 200 × 100 μm area adjacent to a third line, connecting the perpendicular axes at their intersection with the vessel wall (lesion-prone site, A). In a subset of experiments, platelet adhesion was also determined in the proximal portion of the common carotid artery 500 μm upstream of the carotid bifurcation (nonlesion-prone site, B). ACC, common carotid artery; ACE, external carotid artery; ACI, internal carotid artery. (B) Platelet–endothelial cell interactions were investigated in 6-, 8-, 10-, 12-, 16-, and 22-wk-old ApoE −/− mice by in vivo fluorescence microscopy of the common carotid artery in situ. Wild-type animals served as controls. The top and bottom panels summarize transient and firm platelet adhesion, respectively, of 10 experiments per group. Platelets were classified according to their interaction with the endothelial cell lining as described previously (reference 5) and are given per mm2 of vessel surface. (C) The microphotographs show representative in vivo fluorescence microscopy images at distinct time points. White arrows indicate adherent platelets, and the black arrow indicates the area of a fatty streak. The small inserts demonstrate histological sections of the corresponding carotid artery under investigation (original magnification: 200-fold). Underneath, the in vivo microscopic images representative en face carotid arteries, stained with Sudan III, are given. (D) Assessment of platelet adhesion to lesion-prone (white bars) and nonlesion-prone sites (black bars). Platelet adhesion was quantified in the proximal (nonlesion-prone) carotid artery and adjacent to the carotid bifurcation (lesion-prone) in both, young (10-wk-old) and old (22-wk-old) ApoE −/− mice (left). The photomicrograph in the middle shows a representative carotid artery, stained with Sudan III. Atherosclerotic plaque formation occurs preferentially at the carotid bifurcation. Representative histological sections of the carotid bifurcation and the proximal common carotid artery are presented in the right panels (original magnification: 200-fold).
Figure 1.
Platelet adhesion to the endothelium of the common carotid artery in ApoE −/− mice in vivo. (A) Assessment of platelet adhesion at the carotid bifurcation (lesion-prone-site, A) and within the proximal portion of the common carotid artery. For in vivo microscopy, two imaginary perpendicular axes were dropped through the origin of the internal and the external common carotid artery. Platelet and leukocyte adhesion were determined at high magnification (500-fold) in a 200 × 100 μm area adjacent to a third line, connecting the perpendicular axes at their intersection with the vessel wall (lesion-prone site, A). In a subset of experiments, platelet adhesion was also determined in the proximal portion of the common carotid artery 500 μm upstream of the carotid bifurcation (nonlesion-prone site, B). ACC, common carotid artery; ACE, external carotid artery; ACI, internal carotid artery. (B) Platelet–endothelial cell interactions were investigated in 6-, 8-, 10-, 12-, 16-, and 22-wk-old ApoE −/− mice by in vivo fluorescence microscopy of the common carotid artery in situ. Wild-type animals served as controls. The top and bottom panels summarize transient and firm platelet adhesion, respectively, of 10 experiments per group. Platelets were classified according to their interaction with the endothelial cell lining as described previously (reference 5) and are given per mm2 of vessel surface. (C) The microphotographs show representative in vivo fluorescence microscopy images at distinct time points. White arrows indicate adherent platelets, and the black arrow indicates the area of a fatty streak. The small inserts demonstrate histological sections of the corresponding carotid artery under investigation (original magnification: 200-fold). Underneath, the in vivo microscopic images representative en face carotid arteries, stained with Sudan III, are given. (D) Assessment of platelet adhesion to lesion-prone (white bars) and nonlesion-prone sites (black bars). Platelet adhesion was quantified in the proximal (nonlesion-prone) carotid artery and adjacent to the carotid bifurcation (lesion-prone) in both, young (10-wk-old) and old (22-wk-old) ApoE −/− mice (left). The photomicrograph in the middle shows a representative carotid artery, stained with Sudan III. Atherosclerotic plaque formation occurs preferentially at the carotid bifurcation. Representative histological sections of the carotid bifurcation and the proximal common carotid artery are presented in the right panels (original magnification: 200-fold).
Figure 2.
Platelet adhesion precedes leukocyte chemotaxis. (A) Photomicrographs show frozen sections immunostained for VCAM-1 in the time course of atherogenesis (original magnification: 200-fold). (B) Leukocyte adhesion to the endothelium of the common carotid artery was assessed by fluorescence microscopy as described previously (reference 5). The right panel shows representative in vivo fluorescence microscopy images at distinct time points; the data are summarized in the left panel. White arrows indicate adherent leukocytes. Mean ± SEM, n = 10 each group. *P < 0.05.
Figure 2.
Platelet adhesion precedes leukocyte chemotaxis. (A) Photomicrographs show frozen sections immunostained for VCAM-1 in the time course of atherogenesis (original magnification: 200-fold). (B) Leukocyte adhesion to the endothelium of the common carotid artery was assessed by fluorescence microscopy as described previously (reference 5). The right panel shows representative in vivo fluorescence microscopy images at distinct time points; the data are summarized in the left panel. White arrows indicate adherent leukocytes. Mean ± SEM, n = 10 each group. *P < 0.05.
Figure 3.
Platelet membrane glycoproteins GPIbα and GPIIb-IIIa mediate platelet–endothelium adhesion in the common carotid artery of ApoE −/− mice. (A) Platelets were preincubated with blocking mAbs (50 μg/ml) directed against GPIbα (p0p/B-Fab) or GPIIb-IIIa (JON/A-F(ab)2 before they were administered into ApoE −/− mice (10-wk-old). Platelet–endothelial cell interactions were investigated by in vivo fluorescence microscopy (see Materials and Methods). The top panel shows representative photomicrographs, the bottom panel summarizes the data (mean and SEM) of n = 4 experiments. *P < 0.05. (B) Platelets isolated from a Glanzmann's patient with a functional defect in the GPIIb-IIIa receptor (reference 6) or from a healthy control individual were evaluated for platelet–endothelium adhesion in ApoE −/− mice. n = 4, mean ± SEM. *P < 0.05.
Figure 3.
Platelet membrane glycoproteins GPIbα and GPIIb-IIIa mediate platelet–endothelium adhesion in the common carotid artery of ApoE −/− mice. (A) Platelets were preincubated with blocking mAbs (50 μg/ml) directed against GPIbα (p0p/B-Fab) or GPIIb-IIIa (JON/A-F(ab)2 before they were administered into ApoE −/− mice (10-wk-old). Platelet–endothelial cell interactions were investigated by in vivo fluorescence microscopy (see Materials and Methods). The top panel shows representative photomicrographs, the bottom panel summarizes the data (mean and SEM) of n = 4 experiments. *P < 0.05. (B) Platelets isolated from a Glanzmann's patient with a functional defect in the GPIIb-IIIa receptor (reference 6) or from a healthy control individual were evaluated for platelet–endothelium adhesion in ApoE −/− mice. n = 4, mean ± SEM. *P < 0.05.
Figure 4.
Reduction of atherosclerotic lesion formation after inhibition of platelet adhesion by anti-GPIbα treatment. (A) Vascular resistance index (RI) was analyzed by color duplex sonography. Mean ± SEM of 4–6 experiments per group; *P < 0.05. (B) The extension of fatty streaks (μm2) was quantified in common carotid of ApoE −/− mice by Sudan III staining in anti–GPIbα-treated mice and untreated or rat IgG-treated control animals. Data represent mean values with SEM (4–6 experiments per group) of 18-wk-old ApoE −/− mice treated 12 wk with anti-GPIbα mAb. *P < 0.05. (C) Cross-sectional plaque area was assessed on 20 serial section of the common carotid artery adjacent to the carotid bifurcation and was evaluated for each animal as the difference between the area, delimited by the internal elastic lamina, and the lumen area. The results were normalized to total vessel cross-sectional area to eliminate variations due to vessel size. Data represent mean values with SEM (4–6 experiments per group) of ApoE −/− mice treated for 12 wk with anti-GPIbα mAb. *P < 0.05. Representative sections (original magnification: 200-fold) stained with elastica van Giesson reagent are presented in D. ACC, common carotid artery; ACE, external carotid artery, ACI, internal carotid artery. (E) Role of platelet adhesion for atherosclerotic lesion formation in the aortic sinus (top panel) and the right and left main coronary arteries (bottom panel, plaque area is presented as percentage of total cross-sectional intimal area). 18-wk-old ApoE −/− mice were treated with vehicle (Control), irrelevant rat IgG, or anti-GPIbα mAb for 12 wk. Atherosclerotic lesion formation was assessed in the aortic sinus and the proximal coronary arteries. Inhibition of platelet adhesion induced a significant reduction in atherosclerotic lesion formation in the aortic sinus and the proximal coronary arteries. Aortic plaque area is presented in μm2; coronary plaque area is given as percentage of the area delimited by the internal elastic lamina (intimal area). Mean ± SEM, P < 0.05 versus Control. Representative histological sections of the aortic sinus and the coronary arteries stained with elastica van Giesson reagent are presented in F and G, respectively (original magnification: 200-fold).
Figure 4.
Reduction of atherosclerotic lesion formation after inhibition of platelet adhesion by anti-GPIbα treatment. (A) Vascular resistance index (RI) was analyzed by color duplex sonography. Mean ± SEM of 4–6 experiments per group; *P < 0.05. (B) The extension of fatty streaks (μm2) was quantified in common carotid of ApoE −/− mice by Sudan III staining in anti–GPIbα-treated mice and untreated or rat IgG-treated control animals. Data represent mean values with SEM (4–6 experiments per group) of 18-wk-old ApoE −/− mice treated 12 wk with anti-GPIbα mAb. *P < 0.05. (C) Cross-sectional plaque area was assessed on 20 serial section of the common carotid artery adjacent to the carotid bifurcation and was evaluated for each animal as the difference between the area, delimited by the internal elastic lamina, and the lumen area. The results were normalized to total vessel cross-sectional area to eliminate variations due to vessel size. Data represent mean values with SEM (4–6 experiments per group) of ApoE −/− mice treated for 12 wk with anti-GPIbα mAb. *P < 0.05. Representative sections (original magnification: 200-fold) stained with elastica van Giesson reagent are presented in D. ACC, common carotid artery; ACE, external carotid artery, ACI, internal carotid artery. (E) Role of platelet adhesion for atherosclerotic lesion formation in the aortic sinus (top panel) and the right and left main coronary arteries (bottom panel, plaque area is presented as percentage of total cross-sectional intimal area). 18-wk-old ApoE −/− mice were treated with vehicle (Control), irrelevant rat IgG, or anti-GPIbα mAb for 12 wk. Atherosclerotic lesion formation was assessed in the aortic sinus and the proximal coronary arteries. Inhibition of platelet adhesion induced a significant reduction in atherosclerotic lesion formation in the aortic sinus and the proximal coronary arteries. Aortic plaque area is presented in μm2; coronary plaque area is given as percentage of the area delimited by the internal elastic lamina (intimal area). Mean ± SEM, P < 0.05 versus Control. Representative histological sections of the aortic sinus and the coronary arteries stained with elastica van Giesson reagent are presented in F and G, respectively (original magnification: 200-fold).
Figure 4.
Reduction of atherosclerotic lesion formation after inhibition of platelet adhesion by anti-GPIbα treatment. (A) Vascular resistance index (RI) was analyzed by color duplex sonography. Mean ± SEM of 4–6 experiments per group; *P < 0.05. (B) The extension of fatty streaks (μm2) was quantified in common carotid of ApoE −/− mice by Sudan III staining in anti–GPIbα-treated mice and untreated or rat IgG-treated control animals. Data represent mean values with SEM (4–6 experiments per group) of 18-wk-old ApoE −/− mice treated 12 wk with anti-GPIbα mAb. *P < 0.05. (C) Cross-sectional plaque area was assessed on 20 serial section of the common carotid artery adjacent to the carotid bifurcation and was evaluated for each animal as the difference between the area, delimited by the internal elastic lamina, and the lumen area. The results were normalized to total vessel cross-sectional area to eliminate variations due to vessel size. Data represent mean values with SEM (4–6 experiments per group) of ApoE −/− mice treated for 12 wk with anti-GPIbα mAb. *P < 0.05. Representative sections (original magnification: 200-fold) stained with elastica van Giesson reagent are presented in D. ACC, common carotid artery; ACE, external carotid artery, ACI, internal carotid artery. (E) Role of platelet adhesion for atherosclerotic lesion formation in the aortic sinus (top panel) and the right and left main coronary arteries (bottom panel, plaque area is presented as percentage of total cross-sectional intimal area). 18-wk-old ApoE −/− mice were treated with vehicle (Control), irrelevant rat IgG, or anti-GPIbα mAb for 12 wk. Atherosclerotic lesion formation was assessed in the aortic sinus and the proximal coronary arteries. Inhibition of platelet adhesion induced a significant reduction in atherosclerotic lesion formation in the aortic sinus and the proximal coronary arteries. Aortic plaque area is presented in μm2; coronary plaque area is given as percentage of the area delimited by the internal elastic lamina (intimal area). Mean ± SEM, P < 0.05 versus Control. Representative histological sections of the aortic sinus and the coronary arteries stained with elastica van Giesson reagent are presented in F and G, respectively (original magnification: 200-fold).
Figure 4.
Reduction of atherosclerotic lesion formation after inhibition of platelet adhesion by anti-GPIbα treatment. (A) Vascular resistance index (RI) was analyzed by color duplex sonography. Mean ± SEM of 4–6 experiments per group; *P < 0.05. (B) The extension of fatty streaks (μm2) was quantified in common carotid of ApoE −/− mice by Sudan III staining in anti–GPIbα-treated mice and untreated or rat IgG-treated control animals. Data represent mean values with SEM (4–6 experiments per group) of 18-wk-old ApoE −/− mice treated 12 wk with anti-GPIbα mAb. *P < 0.05. (C) Cross-sectional plaque area was assessed on 20 serial section of the common carotid artery adjacent to the carotid bifurcation and was evaluated for each animal as the difference between the area, delimited by the internal elastic lamina, and the lumen area. The results were normalized to total vessel cross-sectional area to eliminate variations due to vessel size. Data represent mean values with SEM (4–6 experiments per group) of ApoE −/− mice treated for 12 wk with anti-GPIbα mAb. *P < 0.05. Representative sections (original magnification: 200-fold) stained with elastica van Giesson reagent are presented in D. ACC, common carotid artery; ACE, external carotid artery, ACI, internal carotid artery. (E) Role of platelet adhesion for atherosclerotic lesion formation in the aortic sinus (top panel) and the right and left main coronary arteries (bottom panel, plaque area is presented as percentage of total cross-sectional intimal area). 18-wk-old ApoE −/− mice were treated with vehicle (Control), irrelevant rat IgG, or anti-GPIbα mAb for 12 wk. Atherosclerotic lesion formation was assessed in the aortic sinus and the proximal coronary arteries. Inhibition of platelet adhesion induced a significant reduction in atherosclerotic lesion formation in the aortic sinus and the proximal coronary arteries. Aortic plaque area is presented in μm2; coronary plaque area is given as percentage of the area delimited by the internal elastic lamina (intimal area). Mean ± SEM, P < 0.05 versus Control. Representative histological sections of the aortic sinus and the coronary arteries stained with elastica van Giesson reagent are presented in F and G, respectively (original magnification: 200-fold).
Figure 4.
Reduction of atherosclerotic lesion formation after inhibition of platelet adhesion by anti-GPIbα treatment. (A) Vascular resistance index (RI) was analyzed by color duplex sonography. Mean ± SEM of 4–6 experiments per group; *P < 0.05. (B) The extension of fatty streaks (μm2) was quantified in common carotid of ApoE −/− mice by Sudan III staining in anti–GPIbα-treated mice and untreated or rat IgG-treated control animals. Data represent mean values with SEM (4–6 experiments per group) of 18-wk-old ApoE −/− mice treated 12 wk with anti-GPIbα mAb. *P < 0.05. (C) Cross-sectional plaque area was assessed on 20 serial section of the common carotid artery adjacent to the carotid bifurcation and was evaluated for each animal as the difference between the area, delimited by the internal elastic lamina, and the lumen area. The results were normalized to total vessel cross-sectional area to eliminate variations due to vessel size. Data represent mean values with SEM (4–6 experiments per group) of ApoE −/− mice treated for 12 wk with anti-GPIbα mAb. *P < 0.05. Representative sections (original magnification: 200-fold) stained with elastica van Giesson reagent are presented in D. ACC, common carotid artery; ACE, external carotid artery, ACI, internal carotid artery. (E) Role of platelet adhesion for atherosclerotic lesion formation in the aortic sinus (top panel) and the right and left main coronary arteries (bottom panel, plaque area is presented as percentage of total cross-sectional intimal area). 18-wk-old ApoE −/− mice were treated with vehicle (Control), irrelevant rat IgG, or anti-GPIbα mAb for 12 wk. Atherosclerotic lesion formation was assessed in the aortic sinus and the proximal coronary arteries. Inhibition of platelet adhesion induced a significant reduction in atherosclerotic lesion formation in the aortic sinus and the proximal coronary arteries. Aortic plaque area is presented in μm2; coronary plaque area is given as percentage of the area delimited by the internal elastic lamina (intimal area). Mean ± SEM, P < 0.05 versus Control. Representative histological sections of the aortic sinus and the coronary arteries stained with elastica van Giesson reagent are presented in F and G, respectively (original magnification: 200-fold).
Figure 5.
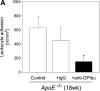
Leukocyte recruitment to the vascular wall of _ApoE_-deficient mice, treated with anti-GPIbα mAb or irrelevant control IgG. Leukocyte accumulation was assessed by in vivo microscopy (A) (4–6 experiments per group) or by CD14 and CD11b mRNA expression in the arterial wall (B). The presence of 18s RNA served as loading control.
Figure 5.
Leukocyte recruitment to the vascular wall of _ApoE_-deficient mice, treated with anti-GPIbα mAb or irrelevant control IgG. Leukocyte accumulation was assessed by in vivo microscopy (A) (4–6 experiments per group) or by CD14 and CD11b mRNA expression in the arterial wall (B). The presence of 18s RNA served as loading control.
Similar articles
- Impact of glycoprotein VI and platelet adhesion on atherosclerosis--a possible role of fibronectin.
Bültmann A, Li Z, Wagner S, Peluso M, Schönberger T, Weis C, Konrad I, Stellos K, Massberg S, Nieswandt B, Gawaz M, Ungerer M, Münch G. Bültmann A, et al. J Mol Cell Cardiol. 2010 Sep;49(3):532-42. doi: 10.1016/j.yjmcc.2010.04.009. Epub 2010 Apr 27. J Mol Cell Cardiol. 2010. PMID: 20430036 - Platelet adhesion via glycoprotein IIb integrin is critical for atheroprogression and focal cerebral ischemia: an in vivo study in mice lacking glycoprotein IIb.
Massberg S, Schürzinger K, Lorenz M, Konrad I, Schulz C, Plesnila N, Kennerknecht E, Rudelius M, Sauer S, Braun S, Kremmer E, Emambokus NR, Frampton J, Gawaz M. Massberg S, et al. Circulation. 2005 Aug 23;112(8):1180-8. doi: 10.1161/CIRCULATIONAHA.105.539221. Epub 2005 Aug 15. Circulation. 2005. PMID: 16103235 - Platelet CD40 Exacerbates Atherosclerosis by Transcellular Activation of Endothelial Cells and Leukocytes.
Gerdes N, Seijkens T, Lievens D, Kuijpers MJ, Winkels H, Projahn D, Hartwig H, Beckers L, Megens RT, Boon L, Noelle RJ, Soehnlein O, Heemskerk JW, Weber C, Lutgens E. Gerdes N, et al. Arterioscler Thromb Vasc Biol. 2016 Mar;36(3):482-90. doi: 10.1161/ATVBAHA.115.307074. Epub 2016 Jan 28. Arterioscler Thromb Vasc Biol. 2016. PMID: 26821950 - Platelets modulate atherogenesis and progression of atherosclerotic plaques via interaction with progenitor and dendritic cells.
Gawaz M, Stellos K, Langer HF. Gawaz M, et al. J Thromb Haemost. 2008 Feb;6(2):235-42. doi: 10.1111/j.1538-7836.2008.02867.x. Epub 2007 Dec 10. J Thromb Haemost. 2008. PMID: 18088342 Review. - Platelets, atherosclerosis and the endothelium: new therapeutic targets?
Tan KT, Lip GY. Tan KT, et al. Expert Opin Investig Drugs. 2003 Nov;12(11):1765-76. doi: 10.1517/13543784.12.11.1765. Expert Opin Investig Drugs. 2003. PMID: 14585053 Review.
Cited by
- Milan PM1 induces adverse effects on mice lungs and cardiovascular system.
Farina F, Sancini G, Longhin E, Mantecca P, Camatini M, Palestini P. Farina F, et al. Biomed Res Int. 2013;2013:583513. doi: 10.1155/2013/583513. Epub 2012 Dec 27. Biomed Res Int. 2013. PMID: 23509745 Free PMC article. - Interplay between inflammation and thrombosis in cardiovascular pathology.
Stark K, Massberg S. Stark K, et al. Nat Rev Cardiol. 2021 Sep;18(9):666-682. doi: 10.1038/s41569-021-00552-1. Epub 2021 May 6. Nat Rev Cardiol. 2021. PMID: 33958774 Free PMC article. Review. - Protein kinase C ε expression in platelets from patients with acute myocardial infarction.
Carubbi C, Mirandola P, Mattioli M, Galli D, Marziliano N, Merlini PA, Lina D, Notarangelo F, Cozzi MR, Gesi M, Ardissino D, De Marco L, Vitale M, Gobbi G. Carubbi C, et al. PLoS One. 2012;7(10):e46409. doi: 10.1371/journal.pone.0046409. Epub 2012 Oct 5. PLoS One. 2012. PMID: 23071564 Free PMC article. - The role of platelets in the recruitment of leukocytes during vascular disease.
Ed Rainger G, Chimen M, Harrison MJ, Yates CM, Harrison P, Watson SP, Lordkipanidzé M, Nash GB. Ed Rainger G, et al. Platelets. 2015;26(6):507-20. doi: 10.3109/09537104.2015.1064881. Epub 2015 Jul 21. Platelets. 2015. PMID: 26196409 Free PMC article. Review. - Silica Nanoparticle-Endothelial Interaction: Uptake and Effect on Platelet Adhesion under Flow Conditions.
Saikia J, Mohammadpour R, Yazdimamaghani M, Northrup H, Hlady V, Ghandehari H. Saikia J, et al. ACS Appl Bio Mater. 2018 Nov 19;1(5):1620-1627. doi: 10.1021/acsabm.8b00466. Epub 2018 Nov 30. ACS Appl Bio Mater. 2018. PMID: 34046558 Free PMC article.
References
- Ross, R. 1999. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 340:115–126. - PubMed
- Frenette, P.S., T.N. Mayadas, H. Rayburn, R.O. Hynes, and D.D. Wagner. 1996. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 84:563–574. - PubMed
- Massberg, S., G. Enders, R. Leiderer, S. Eisenmenger, D. Vestweber, F. Krombach, and K. Messmer. 1998. Platelet-endothelial cell interactions during ischemia/reperfusion: the role of P-selectin. Blood. 92:507–515. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous