Consequences of cellular cholesterol accumulation: basic concepts and physiological implications - PubMed (original) (raw)
Review
Consequences of cellular cholesterol accumulation: basic concepts and physiological implications
Ira Tabas. J Clin Invest. 2002 Oct.
No abstract available
Figures
Figure 1
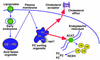
Mechanisms that protect cells from excess accumulation of FC. Lipoprotein-derived cholesterol is distributed to peripheral cellular sites from a putative FC-sorting organelle, which may be a type of late endosome. The npc1 protein is depicted in this organelle as one of the molecules that are known to influence cholesterol trafficking. The cholesterol trafficking itineraries depicted here include transport to ACAT in the endoplasmic reticulum, leading to cholesterol esterification, and to sites of cholesterol efflux in the plasma membrane, leading to removal of cholesterol if appropriate extracellular cholesterol acceptors are present. Not depicted here are those pathways that downregulate the LDL receptor and cholesterol biosynthetic enzymes and those pathways in specialized cells that lead to the metabolism of cholesterol to other molecules, like bile acids and steroid hormones. As described in the text, FC accumulation can occur via inhibition of cholesterol transport to ACAT or to the plasma membrane, or by direct inhibition of ACAT or cholesterol efflux molecules. An increase in neutral cholesteryl esterase (NCEH) activity in the absence of compensatory re-esterification or efflux of cholesterol could also lead to FC accumulation.
Figure 2
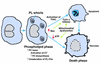
Sequential responses of cultured macrophages to FC loading. In the initial, adaptive phase, CT is activated, leading to increased PC biosynthesis and PC mass and resulting in a normalization of the FC/phospholipid ratio. In addition, there is an increase in the degree of unsaturation of phospholipid fatty acids (FAs). With continued FC loading, death ensues by both apoptotic and necrotic processes. Apoptosis involves both activation of Fas ligand and release of cytochrome c (cyto c) from mitochondria, which may be related to the increased levels of mitochondria-associated Bax in FC-loaded macrophages. FC-induced mitochondrial dysfunction also leads to depletion of cellular ATP stores, which could trigger cellular necrosis.
Figure 3
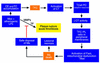
Hypothetical model relating FC loading of lesional macrophages (Mφs) to acute events in advanced atheromata. Progressive FC loading of lesional macrophages leads to a series of phospholipid-related adaptive responses, as described in the text. These adaptive responses eventually fail, leading to macrophage death (see Figure 2). On the one hand, macrophage death may contribute to plaque instability by promoting lesional necrosis. On the other hand, safe disposal of apoptotic macrophages could decrease the number of macrophages that secrete matrix metalloproteinases (MMPs), tissue factor (TF), and inflammatory cytokines. Because these molecules are thought to contribute to plaque rupture and acute thrombosis, macrophage death in this particular context might be protective.
Similar articles
- [Neutral lipid accumulation and phospholipid metabolism in macrophages].
Nishikawa K. Nishikawa K. Tanpakushitsu Kakusan Koso. 1991 Feb;36(3):470-5. Tanpakushitsu Kakusan Koso. 1991. PMID: 2024026 Japanese. No abstract available. - [Peritoneal macrophages as a model for the study of the atherogenic potential of the blood serum].
Tertov VV, Kalenich OS, Orekhov AN. Tertov VV, et al. Ter Arkh. 1990;62(11):102-4. Ter Arkh. 1990. PMID: 2094970 Russian. - Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice.
Zhou J, Lhoták S, Hilditch BA, Austin RC. Zhou J, et al. Circulation. 2005 Apr 12;111(14):1814-21. doi: 10.1161/01.CIR.0000160864.31351.C1. Epub 2005 Apr 4. Circulation. 2005. PMID: 15809369 - [Macrophages and arteriosclerosis].
Yokode M, Kita T. Yokode M, et al. Nihon Naika Gakkai Zasshi. 1994 Sep 10;83(9):1520-5. Nihon Naika Gakkai Zasshi. 1994. PMID: 7798743 Review. Japanese. No abstract available. - [Oxidative stress and atherosclerotic diseases].
Yokode M. Yokode M. Nihon Ronen Igakkai Zasshi. 1997 Sep;34(9):711-5. Nihon Ronen Igakkai Zasshi. 1997. PMID: 9430980 Review. Japanese. No abstract available.
Cited by
- Extracellular Matrix Patches for Endarterectomy Repair.
Allen KB, Adams JD, Badylak SF, Garrett HE, Mouawad NJ, Oweida SW, Parikshak M, Sultan PK. Allen KB, et al. Front Cardiovasc Med. 2021 Feb 11;8:631750. doi: 10.3389/fcvm.2021.631750. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 33644135 Free PMC article. - Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming.
Zimmer S, Grebe A, Bakke SS, Bode N, Halvorsen B, Ulas T, Skjelland M, De Nardo D, Labzin LI, Kerksiek A, Hempel C, Heneka MT, Hawxhurst V, Fitzgerald ML, Trebicka J, Björkhem I, Gustafsson JÅ, Westerterp M, Tall AR, Wright SD, Espevik T, Schultze JL, Nickenig G, Lütjohann D, Latz E. Zimmer S, et al. Sci Transl Med. 2016 Apr 6;8(333):333ra50. doi: 10.1126/scitranslmed.aad6100. Sci Transl Med. 2016. PMID: 27053774 Free PMC article. - Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis.
Savard C, Tartaglione EV, Kuver R, Haigh WG, Farrell GC, Subramanian S, Chait A, Yeh MM, Quinn LS, Ioannou GN. Savard C, et al. Hepatology. 2013 Jan;57(1):81-92. doi: 10.1002/hep.25789. Hepatology. 2013. PMID: 22508243 Free PMC article. - Molecular etiology of atherogenesis--in vitro induction of lipidosis in macrophages with a new LDL model.
Estronca LM, Silva JC, Sampaio JL, Shevchenko A, Verkade P, Vaz AD, Vaz WL, Vieira OV. Estronca LM, et al. PLoS One. 2012;7(4):e34822. doi: 10.1371/journal.pone.0034822. Epub 2012 Apr 13. PLoS One. 2012. PMID: 22514671 Free PMC article. - 7-Ketocholesterol: Effects on viral infections and hypothetical contribution in COVID-19.
Ghzaiel I, Sassi K, Zarrouk A, Nury T, Ksila M, Leoni V, Bouhaouala-Zahar B, Hammami S, Hammami M, Mackrill JJ, Samadi M, Ghrairi T, Vejux A, Lizard G. Ghzaiel I, et al. J Steroid Biochem Mol Biol. 2021 Sep;212:105939. doi: 10.1016/j.jsbmb.2021.105939. Epub 2021 Jun 9. J Steroid Biochem Mol Biol. 2021. PMID: 34118414 Free PMC article. Review.
References
- Dietschy JM, Turley SD, Spady DK. Role of the liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–1659. - PubMed
- Tabas, I., and Kreiger, M. 1999. Lipoprotein receptors and cellular cholesterol metabolism in health and disease. In Molecular basis of heart disease. K.R. Chien, editor. W.B. Saunders Co. New York, New York, USA. 428–457.
- Lin D, et al. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267:1828–1831. - PubMed
- Glass CK, Witztum JL. Atherosclerosis: the road ahead. Cell. 2001;104:503–516. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical