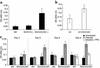Pivotal role of CEACAM1 protein in the inhibition of activated decidual lymphocyte functions - PubMed (original) (raw)
Pivotal role of CEACAM1 protein in the inhibition of activated decidual lymphocyte functions
Gal Markel et al. J Clin Invest. 2002 Oct.
Abstract
Lymphocytes in direct contact with embryonic extravillous trophoblasts constitute more than 40% of decidual cells and appear to play major roles in implantation and early gestation. A unique subset of NK cells, making up 70-80% of decidual lymphocytes, express high levels of CD56 but lack CD16. We have recently demonstrated a novel class I MHC-independent inhibitory mechanism of NK cell cytotoxicity that is mediated by CEACAM1 homotypic interactions. This mechanism is used by some melanoma cells to avoid attack, mainly by CD16(-) NK cells. We now demonstrate that CEACAM1 is expressed on primary extravillous trophoblasts and is upregulated on the vast majority of IL-2-activated decidual lymphocytes, including NK, T, and NKT cells. Importantly, we present evidence that CEACAM1 interactions inhibit the lysis, proliferation, and cytokine secretion of activated decidual NK, T, and NKT cells, respectively. In vivo analysis of decidual lymphocytes isolated from cytomegalovirus-infected (CMV-infected) pregnant women revealed a dramatic increase in the expression of CEACAM1. Finally, we suggest that a novel ligand for this adhesion molecule is present on the surface of CMV-infected fibroblasts. These combined results demonstrate a major role for the CEACAM1 protein in controlling local decidual immune responses.
Figures
Figure 1
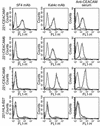
CEACAM1 staining of decidual lymphocytes. Decidual lymphocytes were isolated and quadruple-stained as described in Methods. (a–c) CEACAM1 staining on nonactivated decidual NK cells (a), T cells (b), and NKT cells (c). One representative experiment is shown out of three performed. Decidual lymphocytes were cultured in the presence of IL-2 as described (20) and then screened for CEACAM1 expression with the 5F4 mAb. (d–f) CEACAM1 staining for activated decidual NK clone (d), T clone (e), and NKT clone (f). Similar results were obtained when other lymphocyte clones were used. (g and h) Staining of EVTs for HLA-G and CEACAM1, respectively. Bold lines represent mAb staining and thin lines show background staining.
Figure 2
Staining of .221 cells expressing various members of the CEACAM family using specific anti-CEACAM antibodies. We generated .221 transfectants as described in Methods. Each row shows the staining performed on a particular transfectant (indicated at left), and each column shows the staining with a particular antibody (indicated at top). Bold lines represent antibody staining and thin lines show background staining on .221 cells. One representative experiment is shown out of three performed.
Figure 3
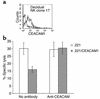
CEACAM1-mediated inhibition of decidual NK cytotoxicity. Decidual NK clones were stained for CEACAM1 expression. (a) CEACAM1 staining of decidual NK clone 17 using the anti-CEACAM1 mAb 5F4 (bold line). The thin line shows the control staining. (b) Killing and inhibition of NK clone 17 by .221 cells and by .221 cells transfected with CEACAM1 (.221/CEACAM1). Blocking experiments were performed using 40 μl/ml of anti-CEACAM antibodies. Average of three independent experiments is shown. Similar results were obtained when other CEACAM1+ NK clones were used.
Figure 4
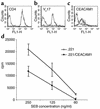
CEACAM1-mediated interactions inhibit SEB-induced T cell proliferation. Decidual T cell clones were tested for expression of CD4 (a), Vβ17 (b), and CEACAM1 (c) by flow cytometry. Bold lines indicate mAb staining and thin lines indicate control staining. (d) Fifty thousand cells of the presented T cell clone were incubated for 2 days with 25,000 irradiated .221 cells or with .221 cells transfected with CEACAM1 (.221/CEACAM1), in the presence of decreasing SEB concentrations as indicated in the figure. Proliferation was measured with 3H-thymidine incorporation. The figure represents the average of ten independent experiments. Similar results were obtained when other T cell clones were used.
Figure 5
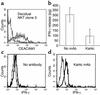
CEACAM1-mediated inhibition of IFN-γ secretion from NKT cells. (a) CEACAM1 expression on isolated activated NKT clone. The bold line shows the staining with 5F4 mAb, and the thin line shows the control staining. (b) The amount of IFN-γ in culture supernatant of mAb-treated and untreated NKT clone cells measured by ELISA. The average of two independent experiments is shown. Cross-linking of surface CEACAM1 was performed without (c) or with (d) the Kat4c mAb, and intracellular staining for IFN-γ was performed. One representative experiment is shown out of two performed. Similar results were obtained when other NKT cell clones were used.
Figure 6
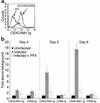
CEACAM1-Ig specifically binds to CMV-infected fibroblasts. (a) Binding of CEACAM1-Ig to .221/CEACAM1 cells (bold line) but not to parental .221 (thin line). The figure shows a representative experiment out of three performed. (b) Day-by-day staining of uninfected and CMV-infected HFF cells in the presence or absence of 300 μg/ml of the antiviral agent PFA. Cells were stained with CEACAM1-Ig and with the control CD99-Ig fusion protein as described in Methods. Data are presented as fold increase above the staining of uninfected cells. The average of two independent experiments is shown.
Figure 7
The functional interactions between BW/CEACAM1ζ and CMV-infected HFFs elicit IL-2 secretion. (a) Spontaneous IL-2 secretion by BW and various BW transfectants after 48 hours of incubation. The average of 20 independent experiments is shown. (b) IL-2 secretion by BW/CEACAM1ζ cells coincubated for 24 hours with irradiated .221 or with .221/CEACAM1 cells. The average of six independent experiments is shown. (c) IL-2 secretion after coincubation of BW or BW/CEACAM1ζ cells with uninfected or CMV-infected HFF cells for 48 hours. No IL-2 secretion above background levels was observed when PFA was included in the assay (only day 6 is shown). Experiments were performed concomitantly with the flow cytometry binding assays of CEACAM1-Ig shown in Figure 5. The average of two independent experiments is shown.
Figure 8
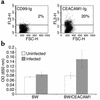
CMV isolated from infected decidua induces a ligand for the CEACAM1 on infected HFF cells. (a) Staining of HFF cells infected with clinical CMV strain with CD99-Ig or with CEACAM1-Ig. No staining was observed when proteins were omitted, indicated by the horizontal line. FSC, forward scatter. (b) IL-2 secretion from BW or BW/CEACAM1ζ cells coincubated with HFF-infected cells for 48 hours. The average of two experiments is shown.
Similar articles
- Differential expression of adhesion and homing molecules by human decidual and peripheral blood lymphocytes in early pregnancy.
Slukvin II, Chernyshov VP, Merkulova AA, Vodyanik MA, Kalinovsky AK. Slukvin II, et al. Cell Immunol. 1994 Oct 1;158(1):29-45. doi: 10.1006/cimm.1994.1254. Cell Immunol. 1994. PMID: 7522129 - The effect of interleukin 2 and transforming growth factor-beta 2 (TGF-beta 2) on the proliferation and natural killer activity of decidual CD16- CD56bright natural killer cells.
Saito S, Morii T, Enomoto M, Sakakura S, Nishikawa K, Narita N, Ichijo M. Saito S, et al. Cell Immunol. 1993 Dec;152(2):605-13. doi: 10.1006/cimm.1993.1316. Cell Immunol. 1993. PMID: 7504983 - Interleukin-8 production by CD16-CD56bright natural killer cells in the human early pregnancy decidua.
Saito S, Kasahara T, Sakakura S, Enomoto M, Umekage H, Harada N, Morii T, Nishikawa K, Narita N, Ichijo M. Saito S, et al. Biochem Biophys Res Commun. 1994 Apr 15;200(1):378-83. doi: 10.1006/bbrc.1994.1459. Biochem Biophys Res Commun. 1994. PMID: 7513162 - The human decidual NK-cell response to virus infection: what can we learn from circulating NK lymphocytes?
Le Bouteiller P, Siewiera J, Casart Y, Aguerre-Girr M, El Costa H, Berrebi A, Tabiasco J, Jabrane-Ferrat N. Le Bouteiller P, et al. J Reprod Immunol. 2011 Mar;88(2):170-5. doi: 10.1016/j.jri.2010.12.005. Epub 2011 Jan 28. J Reprod Immunol. 2011. PMID: 21277025 Review. - [Immune reaction to human cytomegalovirus].
Ito M. Ito M. Nihon Rinsho. 1998 Jan;56(1):62-8. Nihon Rinsho. 1998. PMID: 9465666 Review. Japanese.
Cited by
- High-dimensional mapping of human CEACAM1 expression on immune cells and association with melanoma drug resistance.
Huang YH, Yoon CH, Gandhi A, Hanley T, Castrillon C, Kondo Y, Lin X, Kim W, Yang C, Driouchi A, Carroll M, Gray-Owen SD, Wesemann DR, Drake CG, Bertagnolli MM, Beauchemin N, Blumberg RS. Huang YH, et al. Commun Med (Lond). 2024 Jul 2;4(1):128. doi: 10.1038/s43856-024-00525-8. Commun Med (Lond). 2024. PMID: 38956268 Free PMC article. - Fusobacterium nucleatum CbpF Mediates Inhibition of T Cell Function Through CEACAM1 Activation.
Galaski J, Shhadeh A, Umaña A, Yoo CC, Arpinati L, Isaacson B, Berhani O, Singer BB, Slade DJ, Bachrach G, Mandelboim O. Galaski J, et al. Front Cell Infect Microbiol. 2021 Jul 15;11:692544. doi: 10.3389/fcimb.2021.692544. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34336716 Free PMC article. - The Impact of Preeclampsia on Gene Expression at the Maternal-Fetal Interface.
Winn VD, Gormley M, Fisher SJ. Winn VD, et al. Pregnancy Hypertens. 2011 Jan 1;1(1):100-8. doi: 10.1016/j.preghy.2010.12.001. Pregnancy Hypertens. 2011. PMID: 21743843 Free PMC article. - CEACAM1 structure and function in immunity and its therapeutic implications.
Kim WM, Huang YH, Gandhi A, Blumberg RS. Kim WM, et al. Semin Immunol. 2019 Apr;42:101296. doi: 10.1016/j.smim.2019.101296. Semin Immunol. 2019. PMID: 31604530 Free PMC article. Review. - Novel anti-melanoma immunotherapies: disarming tumor escape mechanisms.
Sapoznik S, Hammer O, Ortenberg R, Besser MJ, Ben-Moshe T, Schachter J, Markel G. Sapoznik S, et al. Clin Dev Immunol. 2012;2012:818214. doi: 10.1155/2012/818214. Epub 2012 Apr 23. Clin Dev Immunol. 2012. PMID: 22778766 Free PMC article. Review.
References
- Bulmer JN, Morrison L, Longfellow A, Riston A, Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod. 1991;6:791–798. - PubMed
- Loke, Y.W., and King, A. 1995. Human implantation: cell biology and immunology. Cambridge University Press. Cambridge, United Kingdom. 1–40.
- King A, et al. On the nature and function of human uterine granular lymphocytes. Immunol Today. 1991;12:432–435. - PubMed
- King A, et al. Recognition of trophoblast HLA class I molecules by decidual NK cell receptors: a review. Placenta. 2000;21(Suppl. A):s81–s85. - PubMed
- King A, et al. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol. 2000;30:1623–1631. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous