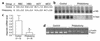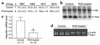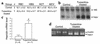The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation - PubMed (original) (raw)
The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation
Gaël Nicolas et al. J Clin Invest. 2002 Oct.
Abstract
The present study was aimed at determining whether hepcidin, a recently identified peptide involved in iron metabolism, plays a role in conditions associated with both iron overload and iron deficiency. Hepcidin mRNA levels were assessed in two models of anemia, acute hemolysis provoked by phenylhydrazine and bleeding provoked by repeated phlebotomies. Hepcidin response to hypoxia was also studied, both ex vivo, in human hepatoma cells, and in vivo. Anemia and hypoxia were associated with a dramatic decrease in liver hepcidin gene expression, which may account for the increase in iron release from reticuloendothelial cells and increase in iron absorption frequently observed in these situations. A single injection of turpentine for 16 hours induced a sixfold increase in liver hepcidin mRNA levels and a twofold decrease in serum iron. The hyposideremic effect of turpentine was completely blunted in hepcidin-deficient mice, revealing hepcidin participation in anemia of inflammatory states. These modifications of hepcidin gene expression further suggest a key role for hepcidin in iron homeostasis under various pathophysiological conditions, which may support the pharmaceutical use of hepcidin agonists and antagonists in various iron homeostasis disorders.
Figures
Figure 1
Hematological indices and hepcidin mRNA levels in phlebotomized mice. (a) Hematological parameters: RBC (106/ml), HGB concentration (g/dl), HCT (%), and MCV (femtoliter [fl]). Results are expressed as mean ± SD for n animals. (b) Hepcidin mRNA content in liver of control and phlebotomized mice as determined by Northern blot analysis. Twenty micrograms of total liver RNAs were electrophoresed, blotted, and hybridized with both hepcidin and 18S-labeled probes (as described in Methods). Autoradiography of a typical experiment. (c) Relative changes in hepcidin mRNA level (18S normalized, arbitrary units) as calculated using a STORM850 PhosphoImager and ImageQuant 5.0. Results are expressed as mean ± SD for n animals, and statistical analysis was performed using the Student t test (unpaired, two-tailed). *P < 0.001. (d) Level of specific hepc1 transcripts in the liver as measured by RT-PCR (as described in Methods). Following PCR, the amplified products (171 bp for hepc1 and 250 bp for β-actin) were separated by electrophoresis on 1.5% agarose gel.
Figure 2
Hematological indices and hepcidin mRNA levels in PHZ-treated mice. (a) Hematological parameters: RBC (106/ml), HGB concentration (g/dl), HCT (%), and MCV (fl). Results are expressed as mean ± SD for n animals. (b) Hepcidin mRNA content in liver of control and PHZ-treated mice as determined by Northern blot analysis. Twenty micrograms of total liver RNAs were electrophoresed, blotted, and hybridized with both hepcidin and 18S-labeled probes (as described in Methods). Autoradiography of a typical experiment. (c) Relative changes in hepcidin mRNA level (18S normalized, arbitrary units) as calculated using a STORM850 PhosphoImager and ImageQuant 5.0. Results are expressed as mean ± SD for n animals, and statistical analysis was performed using the Student t test (unpaired, two-tailed). *P < 0.0001. (d) Level of specific hepc1 transcripts in the liver as measured by RT-PCR (as described in Methods). Following PCR, the amplified products (171 bp for hepc1 and 250 bp for β-actin) were separated by electrophoresis on 1.5% agarose gel.
Figure 3
Effect of PHZ treatment in control mice and experimentally iron-overloaded animals. (a) Hepcidin mRNA content in liver of control mice, mice treated with PHZ, mice experimentally iron overloaded, and mice iron overloaded and treated with PHZ (see Methods) as determined by Northern blot analysis. Twenty micrograms of total liver RNAs were electrophoresed, blotted, and hybridized with both hepcidin and 18S-labeled probes (as described in Methods). Id, iron dextran. (b) Relative changes in hepcidin. mRNA level (18S normalized, arbitrary units) as calculated using a STORM850 PhosphoImager and ImageQuant 5.0. Results are expressed as mean ± SD for n animals, and statistical analysis was performed using the Student t test (unpaired, two-tailed). *P < 0.001 as compared with iron-overloaded mice.
Figure 4
Hepcidin mRNA levels under ex vivo and in vivo hypoxic conditions. (a) Hepcidin mRNA content in HepG2 hepatoma cell lines as determined by Northern blot analysis. HepG2 cells were untreated (20% O2) or treated with 10, 2, or 0.1% O2. Twenty micrograms of total liver RNAs were electrophoresed, blotted, and hybridized with both human hepcidin and 18S-labeled probes (as described in Methods). RNAs from HepG2 cells were also hybridized with a human VEGF cDNA probe as a control of the simulation of the vegf gene under hypoxia. (b) Hepcidin mRNA content in liver of mice housed in hypobaric hypoxic chambers for 1, 2, or 4 days, as determined by Northern blot analysis. Twenty micrograms of total liver RNAs were electrophoresed, blotted, and hybridized with both hepcidin and 18S-labeled probes (as described in Methods). Each lane represented different animals. (c) Level of specific hepc1 transcripts in the liver as measured by RT-PCR (as described in Methods). Following PCR, the amplified products (171 bp for hepc1 and 250 bp for β-actin) were separated by electrophoresis on 1.5 % agarose gel.
Figure 5
Hematological indices and hepcidin mRNA levels in turpentine-treated mice. (a) Hematological parameters in mice following chronic turpentine treatment: RBC (106/ml), HBG concentration (g/dl), HCT (%), and MCV (fl). Results are expressed as mean ± SEM for n animals. (b) Hepcidin mRNA content in liver of control and 16-hour turpentine-treated mice as determined by Northern blot analysis. Twenty micrograms of total liver RNAs were electrophoresed, blotted, and hybridized with a 32P-labeled probe (as described in Methods) for hepcidin. Autoradiography of a typical experiment. (c) Relative changes in hepcidin mRNA level (18S normalized, arbitrary units) as calculated using a STORM850 PhosphoImager and ImageQuant 5.0. Results are expressed as mean ± SD for n animals, and statistical analysis was performed using the Student t test (unpaired, two-tailed). *P < 0.0001. (d) Level of specific hepc1 transcripts in the liver as measured by RT-PCR (as described in Methods). Following PCR, the amplified products (171 bp for hepc1 and 250 bp for β-actin) were separated by electrophoresis on 1.5 % agarose gel.
Figure 6
Effect of a single turpentine injection on serum iron content in wild-type and hepcidin-deficient mice. (a) Serum iron in wild-type mice before (white bar) and after (gray bar) a 16-hour turpentine injection. Results are expressed as mean ± SEM for eight animals. Statistical analysis was performed using Student t test (unpaired, two-tailed). *P < 0.0001. (b) Serum iron in hepcidin-deficient mice before (white bar) and after (gray bar) a 16-hour turpentive injection. Results are expressed as mean ± SEM for seven animals.
Figure 7
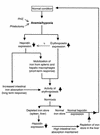
Scheme for suggested role of hepcidin for maintaining iron homeostasis following iron disturbance.
Similar articles
- [An iron regulator hepcidin is affected by EPO].
Sun XF, Zhou DB, Zhao YQ. Sun XF, et al. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006 Aug;14(4):778-82. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006. PMID: 16928320 Chinese. - The iron regulatory peptide hepcidin is expressed in the heart and regulated by hypoxia and inflammation.
Merle U, Fein E, Gehrke SG, Stremmel W, Kulaksiz H. Merle U, et al. Endocrinology. 2007 Jun;148(6):2663-8. doi: 10.1210/en.2006-1331. Epub 2007 Mar 15. Endocrinology. 2007. PMID: 17363462 - Iron metabolism in hepcidin1 knockout mice in response to phenylhydrazine-induced hemolysis.
Masaratana P, Latunde-Dada GO, Patel N, Simpson RJ, Vaulont S, McKie AT. Masaratana P, et al. Blood Cells Mol Dis. 2012 Aug 15;49(2):85-91. doi: 10.1016/j.bcmd.2012.04.003. Epub 2012 May 19. Blood Cells Mol Dis. 2012. PMID: 22609087 - Regulation of iron metabolism by hepcidin.
Nemeth E, Ganz T. Nemeth E, et al. Annu Rev Nutr. 2006;26:323-42. doi: 10.1146/annurev.nutr.26.061505.111303. Annu Rev Nutr. 2006. PMID: 16848710 Review. - [Regulation of body iron homeostasis by hepcidin].
Lipiński P, Starzyński RR. Lipiński P, et al. Postepy Hig Med Dosw (Online). 2004 Mar 8;58:65-73. Postepy Hig Med Dosw (Online). 2004. PMID: 15069380 Review. Polish.
Cited by
- Profiling sirolimus-induced inflammatory syndrome: a prospective tricentric observational study.
Buron F, Malvezzi P, Villar E, Chauvet C, Janbon B, Denis L, Brunet M, Daoud S, Cahen R, Pouteil-Noble C, Gagnieu MC, Bienvenu J, Bayle F, Morelon E, Thaunat O. Buron F, et al. PLoS One. 2013;8(1):e53078. doi: 10.1371/journal.pone.0053078. Epub 2013 Jan 7. PLoS One. 2013. PMID: 23308138 Free PMC article. Clinical Trial. - Iron-hepcidin dysmetabolism, anemia and renal hypoxia, inflammation and fibrosis in the remnant kidney rat model.
Garrido P, Ribeiro S, Fernandes J, Vala H, Bronze-da-Rocha E, Rocha-Pereira P, Belo L, Costa E, Santos-Silva A, Reis F. Garrido P, et al. PLoS One. 2015 Apr 13;10(4):e0124048. doi: 10.1371/journal.pone.0124048. eCollection 2015. PLoS One. 2015. PMID: 25867633 Free PMC article. - Regulation of Iron Metabolism by Hepcidin under Conditions of Inflammation.
Schmidt PJ. Schmidt PJ. J Biol Chem. 2015 Jul 31;290(31):18975-83. doi: 10.1074/jbc.R115.650150. Epub 2015 Jun 8. J Biol Chem. 2015. PMID: 26055723 Free PMC article. Review. - Iron homeostasis in host defence and inflammation.
Ganz T, Nemeth E. Ganz T, et al. Nat Rev Immunol. 2015 Aug;15(8):500-10. doi: 10.1038/nri3863. Epub 2015 Jul 10. Nat Rev Immunol. 2015. PMID: 26160612 Free PMC article. Review. - Red Blood Cell Distribution Width is a Biomarker of Red Cell Dysfunction Associated with High Systemic Inflammation and a Prognostic Marker in Heart Failure and Cardiovascular Disease: A Potential Predictor of Atrial Fibrillation Recurrence.
García-Escobar A, Lázaro-García R, Goicolea-Ruigómez J, González-Casal D, Fontenla-Cerezuela A, Soto N, González-Panizo J, Datino T, Pizarro G, Moreno R, Cabrera JÁ. García-Escobar A, et al. High Blood Press Cardiovasc Prev. 2024 Sep;31(5):437-449. doi: 10.1007/s40292-024-00662-0. Epub 2024 Jul 20. High Blood Press Cardiovasc Prev. 2024. PMID: 39031283 Review.
References
- Finch C. Regulators of iron balance in humans. Blood. 1994;84:1697–1702. - PubMed
- Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin: a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. - PubMed
- Krause A, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–150. - PubMed
- Pigeon C, et al. A new mouse liver specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases