Long-term systemic therapy of Fabry disease in a knockout mouse by adeno-associated virus-mediated muscle-directed gene transfer - PubMed (original) (raw)
Long-term systemic therapy of Fabry disease in a knockout mouse by adeno-associated virus-mediated muscle-directed gene transfer
Hiroshi Takahashi et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Fabry disease is a systemic disease caused by genetic deficiency of a lysosomal enzyme, alpha-galactosidase A (alpha-gal A), and is thought to be an important target for enzyme replacement therapy. We studied the feasibility of gene-mediated enzyme replacement for Fabry disease. The adeno-associated virus (AAV) vector containing the alpha-gal A gene was injected into the right quadriceps muscles of Fabry knockout mice. A time course study showed that alpha-gal A activity in plasma was increased to approximately 25% of normal mice and that this elevated activity persisted for up to at least 30 weeks without development of anti-alpha-gal A antibodies. The alpha-gal A activity in various organs of treated Fabry mice remained 5-20% of those observed in normal mice. Accumulated globotriaosylceramide in these organs was completely cleared by 25 weeks after vector injection. Reduction of globotriaosylceramide levels was also confirmed by immunohistochemical and electronmicroscopic analyses. Echocardiographic examination of treated mice demonstrated structural improvement of cardiac hypertrophy 25 weeks after the treatment. AAV vector-mediated muscle-directed gene transfer provides an efficient and practical therapeutic approach for Fabry disease.
Figures
Figure 1
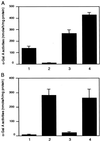
AAV vector-mediated expression of α-gal A in patient's fibroblasts. (A) Fibroblasts from a Fabry patient was transduced with AAV.CAαGBE. α-Gal A activity was determined 3 days after transduction. 1, Nontreated normal fibroblasts; 2, nontreated Fabry fibroblasts; 3, Fabry fibroblasts transduced with 5 × 104 AAV.CAαGBE genomes; 4, Fabry fibroblasts transduced with 1 × 105 AAV.CAαGBE genomes. (B) Fabry fibroblasts were incubated with the conditioned medium (CD) of HeLa cell clone (H#13) secreting α-gal A for overnight. 1, Nontreated fibroblasts; 2, incubation with CD; 3, incubation with CD in the presence of 1 mM mannose-6 phosphate; 4, incubation with CD in the presence of 1 mM glucose-6-phosphate. Values are presented as means ± SD.
Figure 2
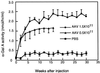
α-Gal A activity in plasma of AAV-treated mice. AAV.CAαGBE (1.5 × 1011 or 0.5 × 1011 vector genomes) was injected into the right quadriceps muscle of 12-week-old Fabry mice. α-Gal A activity in plasma was determined every 2 weeks. Values are expressed as the mean ± SD (n = 3).
Figure 3
PCR analysis of the transduced α-gal A gene in Fabry mice. DNA was extracted from organs of Fabry mice 25 weeks after vector injection and analyzed by PCR. The 177-bp fragment corresponds to the human α-gal A cDNA derived from AAV.CAαGBE, whereas the 1.3-kb fragment corresponds to the mouse genomic α-gal A gene. Integrity of DNA was determined by amplifying a 604-bp region of the murine β-actin gene, using appropriate primers. 1, Heart; 2, kidney; 3, liver; 4, right muscle; 5, left muscle; 6, brain; 7, spleen; 8, lung; 9, positive control.
Figure 4
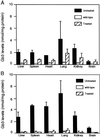
Gb3 levels in organs of treated Fabry mice. Gb3 levels of various organs of treated and untreated Fabry mice, and age-matched wild-type mice at 15 (A) and 25 (B) weeks postinjection. Values are expressed as the mean ± SD (n = 3).
Figure 5
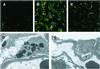
Histological examination of kidneys of treated Fabry mice. (A_–_C) Immunohistochemical staining of kidney tissues from a wild-type mouse (A), an untreated Fabry mouse (B), and a treated Fabry mouse 25 weeks after vector injection (C) were immunostained with a monoclonal anti-Gb3 antibody, followed by FITC-labeled anti-mouse IgG. (D and E) Electron microscopic analysis of the kidney. Lipid inclusions with electron-dense concentric lamellar structures in glomerular epithelial cells were observed in the kidneys of untreated Fabry mice (D). The lipid inclusions were cleared 32 weeks after treatment with the AAV vector (E).
Similar articles
- Adeno-associated viral vector-mediated gene transfer results in long-term enzymatic and functional correction in multiple organs of Fabry mice.
Jung SC, Han IP, Limaye A, Xu R, Gelderman MP, Zerfas P, Tirumalai K, Murray GJ, During MJ, Brady RO, Qasba P. Jung SC, et al. Proc Natl Acad Sci U S A. 2001 Feb 27;98(5):2676-81. doi: 10.1073/pnas.051634498. Proc Natl Acad Sci U S A. 2001. PMID: 11226298 Free PMC article. - Partial correction of the alpha-galactosidase A deficiency and reduction of glycolipid storage in Fabry mice using synthetic vectors.
Przybylska M, Wu IH, Zhao H, Ziegler RJ, Tousignant JD, Desnick RJ, Scheule RK, Cheng SH, Yew NS. Przybylska M, et al. J Gene Med. 2004 Jan;6(1):85-92. doi: 10.1002/jgm.468. J Gene Med. 2004. PMID: 14716680 - Preselective gene therapy for Fabry disease.
Qin G, Takenaka T, Telsch K, Kelley L, Howard T, Levade T, Deans R, Howard BH, Malech HL, Brady RO, Medin JA. Qin G, et al. Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):3428-33. doi: 10.1073/pnas.061020598. Epub 2001 Mar 6. Proc Natl Acad Sci U S A. 2001. PMID: 11248095 Free PMC article. - [Gene therapy of Gaucher's and Fabry's diseases: current status and prospects].
Fabrega S, Lehn P. Fabrega S, et al. J Soc Biol. 2002;196(2):175-81. J Soc Biol. 2002. PMID: 12360746 Review. French. - [Fabry's disease (alpha-galactosidase-A deficiency): recent therapeutic innovations].
Germain DP. Germain DP. J Soc Biol. 2002;196(2):183-90. J Soc Biol. 2002. PMID: 12360747 Review. French.
Cited by
- α-Galactosidase A Augmentation by Non-Viral Gene Therapy: Evaluation in Fabry Disease Mice.
Rodríguez-Castejón J, Alarcia-Lacalle A, Gómez-Aguado I, Vicente-Pascual M, Solinís Aspiazu MÁ, Del Pozo-Rodríguez A, Rodríguez-Gascón A. Rodríguez-Castejón J, et al. Pharmaceutics. 2021 May 21;13(6):771. doi: 10.3390/pharmaceutics13060771. Pharmaceutics. 2021. PMID: 34064206 Free PMC article. - Preclinical evaluation of FLT190, a liver-directed AAV gene therapy for Fabry disease.
Jeyakumar JM, Kia A, Tam LCS, McIntosh J, Spiewak J, Mills K, Heywood W, Chisari E, Castaldo N, Verhoef D, Hosseini P, Kalcheva P, Cocita C, Miranda CJ, Canavese M, Khinder J, Rosales C, Hughes D, Sheridan R, Corbau R, Nathwani A. Jeyakumar JM, et al. Gene Ther. 2023 Jun;30(6):487-502. doi: 10.1038/s41434-022-00381-y. Epub 2023 Jan 11. Gene Ther. 2023. PMID: 36631545 Free PMC article. - Multimodal photoacoustic ophthalmoscopy in mouse.
Song W, Wei Q, Feng L, Sarthy V, Jiao S, Liu X, Zhang HF. Song W, et al. J Biophotonics. 2013 Jun;6(6-7):505-512. doi: 10.1002/jbio.201200061. Epub 2012 May 31. J Biophotonics. 2013. PMID: 22649053 Free PMC article. - Treatment of Fabry Disease: Established and Emerging Therapies.
Umer M, Kalra DK. Umer M, et al. Pharmaceuticals (Basel). 2023 Feb 20;16(2):320. doi: 10.3390/ph16020320. Pharmaceuticals (Basel). 2023. PMID: 37259462 Free PMC article. Review. - Promise of adeno-associated virus as a gene therapy vector for cardiovascular diseases.
Bera A, Sen D. Bera A, et al. Heart Fail Rev. 2017 Nov;22(6):795-823. doi: 10.1007/s10741-017-9622-7. Heart Fail Rev. 2017. PMID: 28589503 Review.
References
- Brady R O, Gal A E, Bradley R M, Martensson E, Warshaw A L, Laster L. N Engl J Med. 1967;276:1163–1167. - PubMed
- Desnick R J, Ioannou Y A, Eng C M. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 2001. pp. 3733–3774.
- Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, Yoshida A, Kuriyama M, Hayashibe H, Sakuraba H, et al. N Engl J Med. 1995;333:288–293. - PubMed
- Brady R O, Tallman J F, Johnson W G, Gal A E, Leahy W R, Quirk J M, Dekaban A S. N Engl J Med. 1973;289:9–14. - PubMed
- Von Figura K, Hasilik A. Annu Rev Biochem. 1986;55:167–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases