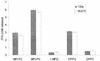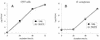Characterization of the gene encoding the major secreted lysophospholipase A of Legionella pneumophila and its role in detoxification of lysophosphatidylcholine - PubMed (original) (raw)
Characterization of the gene encoding the major secreted lysophospholipase A of Legionella pneumophila and its role in detoxification of lysophosphatidylcholine
Antje Flieger et al. Infect Immun. 2002 Nov.
Abstract
We previously showed that Legionella pneumophila secretes, via its type II secretion system, phospholipase A activities that are distinguished by their specificity for certain phospholipids. In this study, we identified and characterized plaA, a gene encoding a phospholipase A that cleaves fatty acids from lysophospholipids. The plaA gene encoded a 309-amino-acid protein (PlaA) which had homology to a group of lipolytic enzymes containing the catalytic signature GDSL. In Escherichia coli, the cloned gene conferred trypsin-resistant hydrolysis of lysophosphatidylcholine and lysophosphatidylglycerol. An L. pneumophila plaA mutant was generated by allelic exchange. Although the mutant grew normally in standard buffered yeast extract broth, its culture supernatants lost greater than 80% of their ability to release fatty acids from lysophosphatidylcholine and lysophosphatidylglycerol, implying that PlaA is the major secreted lysophospholipase A of L. pneumophila. The mutant's reduced lipolytic activity was confirmed by growth on egg yolk agar and thin layer chromatography and was complemented by reintroduction of an intact copy of plaA. Overexpression of plaA completely protected L. pneumophila from the toxic effects of lysophosphatidylcholine, suggesting a role for PlaA in bacterial detoxification of lysophospholipids. The plaA mutant grew like the wild type in U937 cell macrophages and Hartmannella vermiformis amoebae, indicating that PlaA is not essential for intracellular infection of L. pneumophila. In the course of characterizing plaA, we discovered that wild-type legionellae secrete a phospholipid cholesterol acyltransferase activity, highlighting the spectrum of lipolytic enzymes produced by L. pneumophila.
Figures
FIG. 1.
Model of the two-step hydrolysis of phosphatidylcholine by secreted PLA and secreted lysophospholipase A (LPLA) activities of L. pneumophila.
FIG. 2.
The plaA locus in L. pneumophila and recombinant E. coli. The upper line represents a 5-kb region of the L. pneumophila chromosome that contains the lysophospholipase A gene (plaA), along with the location of relevant restriction enzyme sites. The dashed line represents the DNA region that we sequenced and have deposited in the GenBank database. The arrows below this line depict the relative location, size, and orientation of plaA and neighboring genes. The thick lines at the bottom of the figure represent the segments of Legionella DNA that were cloned into plasmid vectors. Plasmid pAF3 contained a Kmr gene cassette in place of the Legionella sequences that normally exist between the indicated _Bst_98I sites. The + and − symbols denote whether supernatants from the recombinant E. coli exhibited increased lysophospholipase A activity.
FIG. 3.
Sequence alignment of L. pneumophila PlaA with members of the GDSL family. Sequences of the 12 closest matches to PlaA are aligned along the five conserved blocks of the GDSL family. An asterisk designates those positions where an amino acid is conserved in at least six of the homologs. The amino acids comprising the putative catalytic triad of PlaA are shown in italics, and the residues conserved in the SGNH family are underlined. The digits before and after blocks I to V indicate the number of amino acid residues present before and after the conserved regions.
FIG. 4.
Lysophospholipase A activity of E. coli containing L. pneumophila plaA. Culture supernatants of E. coli NovaBlue containing pBluescript KS(+) (pBlue) or its derivatives pAF2 or pAF3 as well as E. coli DH5α harboring pMMB207 or its derivative pAF8 were mixed with MPLPC (A) or MPLPG (B) in the presence or absence of trypsin and, after a 5-h incubation at 37°C, the release of FFA was quantified. Data are expressed as differences between the amount of FFA released by the culture supernatant and the amount released by uninoculated LB broth. The results represent the means ± standard deviations of duplicate cultures and are representative of three independent experiments. Asterisks denote significant differences in lysophospholipase activity between E. coli containing plaA and the respective vector control (P < 0.05; Student's t test).
FIG. 5.
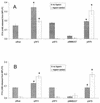
Lipolytic activities of culture supernatants of wild-type and plaA mutant L. pneumophila. Culture supernatants from late log phase of BYE cultures of strains 130b and NU270 were incubated with MPLPC, MPLPG, 1-MPG, DPPG, or DPPC for 2.5 h at 37°C, and then the release of FFA was quantified. Data are expressed as differences between the amount of FFA released by the culture supernatant and the amount released by uninoculated BYE broth. The results represent the means ± standard deviations of triplicate cultures and are representative of two independent experiments. Asterisks denote significant differences in lipolytic activities between wild-type L. pneumophila and NU270 (P < 0.01 for MPLPC, MPLPG, DPPG, and DPPC hydrolysis, and P < 0.05 for hydrolysis of 1-MPG; Student's t test).
FIG. 6.
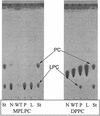
TLC analysis of lipid hydrolysis by wild-type and mutant L. pneumophila. Tenfold-concentrated culture supernatants from late log phase of strains 130b (WT), NU270 (P), and an lspDE mutant (L) were incubated with MPLPC or DPPC for 18 h at 37°C. Subsequently, the lipids were extracted and separated by TLC. A mixture of concentrated BYE broth and the lipids was also incubated and served as a negative control (N). In the case of incubations with MPLPC (left panel), the samples were examined for the degradation of the lipid substrate and, in the cases of incubations with DPPC, for enrichment of lysophosphatidylcholine (LPC). For the qualitative identification of the lipid spots, lanes containing MPLPC and DPPC standards are marked St. The observations depicted here were made on at least two occasions.
FIG. 7.
Lipolytic activities of cell lysates of wild-type and plaA mutant L. pneumophila. Cell lysates from late log phase BYE cultures of strains 130b and NU270 were incubated with MPLPC, MPLPG, 1-MPG, DPPG, or DPPC for 2.5 h at 37°C. Subsequently, the release of FFA was quantified. Data are expressed as differences between the amount of FFA released by the culture supernatant and the amount released by uninoculated BYE broth that, like the cell samples, had been treated with lysozyme and Triton X-100. Results represent the means ± standard deviations of triplicate cultures and are representative of two independent experiments.
FIG. 8.
Effect of MPLPC on the viability of wild-type and mutant L. pneumophila. Strains 130b, plaA mutant NU270, and lspDE mutant NU258 were inoculated into BYE broth at an OD660 of 0.2 to 0.3 and grown at 37°C with shaking. (A) When the cultures reached mid-log phase (arrow), 0.2 mM MPLPC (⋄, □, ○) or medium control (♦, ▪, •) was added. Bacterial growth was monitored by recording the cultures' OD660. (B) After 16 h of incubation with MPLPC, cultures were serially diluted and plated on BCYE agar for determination of CFU. Wild-type and plaA mutant L. pneumophila containing pMMB207 or pAF8 were treated as described above. (C) Bacteria were plated for determination of CFU after 4 h of incubation with 0.2 mM MPLPC. Data represent the means ± standard deviations of triplicate cultures and are representative of two independent experiments. One asterisk designates significant differences between L. pneumophila cultures untreated versus treated with MPLPC (P < 0.05; Student's t test). An additional asterisk denotes significant differences between the wild type and the plaA mutant or between the wild type and the lspDE mutant (P < 0.05; Student's t test).
FIG. 9.
Lipolytic activities of culture supernatants of wild-type and proA mutant L. pneumophila. Culture supernatants from log-phase cultures of strains 130b and proA mutant AA200 were incubated with MPLPC, MPLPG, 1-MPG, DPPG, or DPPC for 2.5 h at 37°C. Subsequently, the release of FFA was quantified. Data are expressed as differences between the amount of FFA released by the culture supernatant versus the amount released by uninoculated BYE broth. Results represent the means ± standard deviations of four cultures and are representative of two independent experiments. Asterisks denote significant differences in lipolytic activities between wild-type L. pneumophila and the proA mutant (P < 0.05; Student's t test).
FIG. 10.
GCAT activity of culture supernatants from wild-type and plaA mutant L. pneumophila. (A) Twenty-fold-concentrated culture supernatants of strains 130b (WT) incubated with mixtures of DPPG, DPPC, MPLPC, MPLPG, or 1-MPG with cholesterol. (B) Twenty-fold-concentrated culture supernatants of strains 130b (WT) and NU270 (P) incubated with mixtures of DPPG or MPLPC with cholesterol for 18 h at 37°C. Subsequently, the lipids were extracted and separated by TLC. A mixture of concentrated BYE broth and the lipids served as a negative control (N). The samples were examined for generation of cholesterol esters as a measure of GCAT activity. Lanes containing cholesterol palmitate (CE), tripalmitoylglycerol (TG), palmitic acid (FFA), and cholesterol (C) standards are marked St. The white arrows in panel A indicate cholesterol esters formed by L. pneumophila GCAT activity. The black arrows indicate the position of unknown polar compounds produced by L. pneumophila concentrated supernatants. Data are representative of two independent experiments.
FIG. 11.
Intracellular infection by wild-type and plaA mutant L. pneumophila. Strains 130b and NU270 were used to infect monolayers of U937 macrophages (A) or cultures of H. vermiformis amoebae (B) at a multiplicity of infection of 0.1. At 0, 24, 48, and 72 h postinoculation, the numbers of bacteria were quantitated by plating aliquots on BCYE agar. Results represent the means ± standard deviations of triplicate samples and are representative of two independent experiments.
Similar articles
- Characterization of the major secreted zinc metalloprotease- dependent glycerophospholipid:cholesterol acyltransferase, PlaC, of Legionella pneumophila.
Banerji S, Bewersdorff M, Hermes B, Cianciotto NP, Flieger A. Banerji S, et al. Infect Immun. 2005 May;73(5):2899-909. doi: 10.1128/IAI.73.5.2899-2909.2005. Infect Immun. 2005. PMID: 15845496 Free PMC article. - Cloning and characterization of the gene encoding the major cell-associated phospholipase A of Legionella pneumophila, plaB, exhibiting hemolytic activity.
Flieger A, Rydzewski K, Banerji S, Broich M, Heuner K. Flieger A, et al. Infect Immun. 2004 May;72(5):2648-58. doi: 10.1128/IAI.72.5.2648-2658.2004. Infect Immun. 2004. PMID: 15102773 Free PMC article. - Novel lysophospholipase A secreted by Legionella pneumophila.
Flieger A, Gong S, Faigle M, Stevanovic S, Cianciotto NP, Neumeister B. Flieger A, et al. J Bacteriol. 2001 Mar;183(6):2121-4. doi: 10.1128/JB.183.6.2121-2124.2001. J Bacteriol. 2001. PMID: 11222614 Free PMC article. - The manifold phospholipases A of Legionella pneumophila - identification, export, regulation, and their link to bacterial virulence.
Banerji S, Aurass P, Flieger A. Banerji S, et al. Int J Med Microbiol. 2008 Apr;298(3-4):169-81. doi: 10.1016/j.ijmm.2007.11.004. Epub 2008 Jan 4. Int J Med Microbiol. 2008. PMID: 18178130 Review. - Secreted phospholipases of the lung pathogen Legionella pneumophila.
Hiller M, Lang C, Michel W, Flieger A. Hiller M, et al. Int J Med Microbiol. 2018 Jan;308(1):168-175. doi: 10.1016/j.ijmm.2017.10.002. Epub 2017 Oct 28. Int J Med Microbiol. 2018. PMID: 29108710 Review.
Cited by
- Surface translocation by Legionella pneumophila: a form of sliding motility that is dependent upon type II protein secretion.
Stewart CR, Rossier O, Cianciotto NP. Stewart CR, et al. J Bacteriol. 2009 Mar;191(5):1537-46. doi: 10.1128/JB.01531-08. Epub 2008 Dec 29. J Bacteriol. 2009. PMID: 19114479 Free PMC article. - Structural and functional characterisation of TesA - a novel lysophospholipase A from Pseudomonas aeruginosa.
Kovačić F, Granzin J, Wilhelm S, Kojić-Prodić B, Batra-Safferling R, Jaeger KE. Kovačić F, et al. PLoS One. 2013 Jul 18;8(7):e69125. doi: 10.1371/journal.pone.0069125. Print 2013. PLoS One. 2013. PMID: 23874889 Free PMC article. - SseJ deacylase activity by Salmonella enterica serovar Typhimurium promotes virulence in mice.
Ohlson MB, Fluhr K, Birmingham CL, Brumell JH, Miller SI. Ohlson MB, et al. Infect Immun. 2005 Oct;73(10):6249-59. doi: 10.1128/IAI.73.10.6249-6259.2005. Infect Immun. 2005. PMID: 16177296 Free PMC article. - The hemolytic and cytolytic activities of Serratia marcescens phospholipase A (PhlA) depend on lysophospholipid production by PhlA.
Shimuta K, Ohnishi M, Iyoda S, Gotoh N, Koizumi N, Watanabe H. Shimuta K, et al. BMC Microbiol. 2009 Dec 16;9:261. doi: 10.1186/1471-2180-9-261. BMC Microbiol. 2009. PMID: 20003541 Free PMC article. - The type II protein secretion system of Legionella pneumophila promotes growth at low temperatures.
Söderberg MA, Rossier O, Cianciotto NP. Söderberg MA, et al. J Bacteriol. 2004 Jun;186(12):3712-20. doi: 10.1128/JB.186.12.3712-3720.2004. J Bacteriol. 2004. PMID: 15175284 Free PMC article.
References
- Aragon, V., S. Kurtz, O. Rossier, and N. P. Cianciotto. 2002. Legionella pneumophila genes that encode lipase and phospholipase C activities. Microbiology 148:2223-2231. - PubMed
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1989. Current protocols in molecular biology. Wiley, New York, N.Y.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources