A receptor for activated C kinase is part of messenger ribonucleoprotein complexes associated with polyA-mRNAs in neurons - PubMed (original) (raw)
A receptor for activated C kinase is part of messenger ribonucleoprotein complexes associated with polyA-mRNAs in neurons
Frank Angenstein et al. J Neurosci. 2002.
Abstract
Long-lasting changes in synaptic functions after an appropriate stimulus require altered protein expression at the synapse. To restrict changes in protein composition to activated synapses, proteins may be synthesized locally as a result of transmitter receptor-triggered signaling pathways. Second messenger-controlled mechanisms that affect mRNA translation are essentially unknown. Here we report that a receptor for activated C kinase, RACK1, is a component of messenger ribonucleoprotein (mRNP) complexes. RACK1 is predominantly associated with polysome-bound, polyA-mRNAs that are being actively translated. We find it to be present in a complex with beta-tubulin and at least two mRNA-binding proteins, polyA-binding protein 1 and a 130 kDa polyA-mRNA binding protein (KIAA0217). Activation of PKCbeta2 in vitro by phosphatidylserine/diacylglycerol or in hippocampal slices by metabotropic glutamate receptor stimulation increased the amount of RACK1/PKCbeta2 associated with polysome-bound polyA-mRNAs. In vitro, PKCbeta2 can phosphorylate a subset of polyA-mRNA-associated proteins that are also phosphorylated under in vivo conditions. On the basis of these findings plus the somatodendritic localization of RACK1, we hypothesize that metabotropic glutamate receptor-triggered binding of activated PKCbeta2 to mRNP complexes bound to polyA-mRNAs is involved in activity-triggered control of protein synthesis.
Figures
Fig. 1.
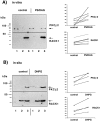
Preparation of mRNPs and detection of RACK1.A, Nontranslated mRNAs were separated from translated mRNAs by discontinuous sucrose density gradient centrifugation. Separation of the resulting interfaces and pellet by a 15–45% continuous sucrose gradient revealed that free mRNA/mRNP complexes and particles smaller than 40S were enriched in the first interface and free ribosomal subunits and monosomes were enriched in the second (12%–33% sucrose density) interface, whereas polysomes were found in the 33% sucrose pellet (right side). B, PolyA-mRNAs were purified from each fraction by binding to oligo(dT)-cellulose and, together with copurified proteins, eluted with 10 m
m
Tris/HCl, separated by SDS-PAGE, and silver-stained.C, Western blot analysis of the same samples using monoclonal antibodies recognizing RACK1, FMRP, PABP1 (location indicated by an arrow), and G3BP-2a. 1, Fraction 1; 2, fraction 2; 3, fraction 3.
Fig. 2.
Comparison of oligo(dT)-cellulose bound proteins with proteins that can be eluted with polyA-mRNAs by 10 m
m
Tris/HCl. A postmitochondrial supernatant was stimulated with PS/DAG, and oligo(dT)-cellulose bound proteins were prepared from both interfaces and the 33% sucrose pellet and released either by SDS-sample buffer (4% SDS, 250 m
m
Tris, 50 m
m
DTT, 3 m
m
EDTA, 20% glycerol, pH 8.0) or by 10 m
m
Tris/HCl, pH 7.5. Although a number of proteins bind to oligo(dT)-cellulose, only a fraction of them can be eluted together with polyA-mRNAs by 10 m
m
Tris/HCl (silver stain). Thus, PKCβ2 (indicated by an arrow) binds probably nonspecifically to oligo(dT)-cellulose in fraction 1 because it cannot be released by 10 m
m
Tris/HCl; in contrast, the kinase can be coreleased with polyA-mRNAs in fraction 3. Comparable with the location of PKCβ2, RACK1 (location shown by an_arrow_) can be released from oligo(dT)-cellulose by 10 m
m
Tris/HCl from the fraction corresponding to translated polyA-mRNAs.
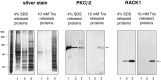
Fig. 3.
Binding of RACK1 to oligo(dT)-cellulose is mediated by polyA-mRNAs. A, PolyA and polyU oligonucleotides compete with the binding of RACK1 to oligo(dT)-cellulose. The locations of PABP1 and RACK1 in a silver-stained gel, as identified by mass spectrometry, are indicated by an arrow. Preincubation of oligo(dT)-cellulose with polyA (A) or incubation of the sample with polyU (U) during the binding resulted in decreased binding of a number of proteins, including PABP1 and RACK1, relative to control preparations (C). In contrast, the presence of polyG (G) did not affect the binding of PABP1 and RACK1 to oligo(dT)-cellulose. B, RNase A (10 μg/ml) treatment abolished the binding of RACK1 to oligo(dT)-cellulose. Under control conditions (top,first 3 lanes), RACK1 can be captured with oligo(dT)-cellulose from fraction 3 (Figs. 1, 2), which includes translated mRNAs. Treatment of these fractions with RNase A resulted in a complete absence of RACK1 among the proteins that are bound to oligo(dT)-cellulose (top, last 3 lanes).C, RACK1 can be released with polyA-mRNAs from both oligo(dT)-cellulose (first 3 lanes) and polyU-agarose (last 3 lanes). PolyA-mRNA/mRNP complexes were prepared from each fraction and incubated with oligo(dT)-cellulose or polyU-agarose and released with 10 m
m
Tris/HCl, pH 7.5, further indicating that RACK1 binds via polyA-mRNA and not nonspecifically to cellulose.
Fig. 4.
RACK1 is preferentially associated with translated mRNAs. Seventeen fractions of a continuous sucrose gradient (15–45%) were collected, and those containing polyA-mRNAs/mRNP complexes were purified using oligo(dT)-cellulose. The presence of RACK1 and PABP1 (indicated by arrows) was determined by Western blot assay. For that the blot was cut (at ∼55 kDa); the top part was stained for PABP1 and the lower part for RACK1. A, Under the control condition, PABP1 was detectable in all fractions, whereas RACK1 appeared in the fraction that contained 40S and heavier particles (fractions 4_-17).B, Absence of Mg2+ within the sucrose gradient caused a complete dissociation of polysomes, and therefore only one peak containing free ribosomal subunits was detectable. Under this condition RACK1 was not detectable in fractions higher than_11. The distribution of PABP1 changed in a similar way, except that we could detect PABP1 in fraction 17, which might correspond to translationally arrested RNA granules (Krichevsky and Kosik, 2001).
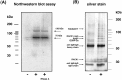
Fig. 5.
RACK1 is in a complex with at least two polyA-mRNA binding proteins. A, To determine whether RACK1 binds directly or via another protein to polyA-mRNAs, a Northwestern blot analysis was performed. RACK1 and coimmunoprecipitated proteins were blotted on nitrocellulose, renatured, and incubated with32P-labeled polyA-mRNAs [−, immunoprecipitation was performed without the RACK1 IgM-mAb (negative control); +, immunoprecipitation with RACK1 IgM-mAb]. Two proteins, with an apparent molecular weight of 75 kDa (Rimb1) and 130 kDa (Rimb2), were found to bind polyA-mRNAs. RACK1, at ∼32 kDa, did not bind polyA-mRNAs in this assay. The presence of RNase A (last lane) did not prevent the interaction of these proteins with RACK1. B, To identify the mRNA-binding proteins, RACK1-IgM coimmunoprecipitated proteins were released from the agarose beads by 0.1
m
glycine, pH 3.0, and silver-stained. The 75 kDa (Rimb1) and 130 kDa (Rimb2) bands were cut out and analyzed by mass spectrometry. The Rimb1 protein band contained (1) the polyA-binding protein (PABP1), (2) HSP70, and (3) the Ras-GTPase-activating protein (GAP120) SH3-domain-binding protein 2a (G3BP-2a) (Table 2). The Rimb2 protein band was found to contain the protein KIAA0217, a protein with an RNA-binding domain (Nagase et al., 1996), and a GPI-anchored protein (Table 3).
Fig. 6.
RACK1 coimmunoprecipitates with PABP1.A, RACK1 was immunoprecipitated from the protein fraction that was released from a polysomal pellet by 30 m
m
EDTA with two different monoclonal antibodies [left side, clone B-3, an IgG2a (Santa Cruz Biotechnology);right side, clone 20, an IgM (Transduction Laboratories)]. The immunoprecipitate was separated using SDS-PAGE and silver-stained. In confirmation of a previous experiment (Fig. 5), the additional protein at 75 kDa (left side,arrow) that was not detectable in the negative control (−, immunoprecipitation protocol using only the protein fraction but without RACK1 mAb) was identified by mass spectrometry as the PABP1 and G3BP-2a, and the 130 kDa protein was identified as KIAA0217 and a GPI-anchored protein. An asterisk indicates the localization of the added RNase A. B, An aliquot of the same sample used for silver staining was blotted onto nitrocellulose and immunostained [left 3 blots correspond to the RACK1-IgG immunoprecipitation (IP); right 2 blots correspond to RACK1-IgM IP]. By confirming the mass spectrometry results, we could detect both PABP1 and G3BP-2a as proteins that coimmunoprecipitate with RACK1 by Western blot assay.C, Negative control (immunoprecipitation without addition of a RACK1-mAb); mab, precipitation of the RACK1-mAb in the absence of the sample. The secondary antibody stained nonspecifically the RNase A (asterisk). The interaction between RACK1 and PABP1 was not abolished by treatment of the immunoprecipitate with 10 U/ml RNase A for 20 min at room temperature. In contrast, there was no coimmunoprecipitation of G3BP-2a when RNase A was added, indicating that both proteins are linked via mRNA to each other. RACK1 staining (arrow) checked the efficiency of the immunoprecipitation.
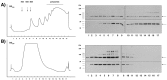
Fig. 7.
Stimulation of PKCβ2 by phosphatidylserine/diacylglycerol (PS/DAG) causes an association with mRNPs associated with translated mRNAs.A, Activation of PKC by application of phosphatidylserine/diacyglycerol to a postmitochondrial supernatant resulted in an increased amount of RACK1 and PKCβ2 in fraction_3_ that could be released from oligo(dT)-cellulose by 10 m
m
Tris/HCl (polysome-bound mRNA/mRNP-complexes).Left, The amount of PKCβ2 and RACK1 associated with mRNAs was determined by Western blot assay; the blot was cut vertically, and the top part used for PKCβ2 staining and the bottom part for RACK1 staining (1, 2, and_3_ indicate the fraction of nontranslated mRNA/mRNP complexes, mRNA/mRNP complexes associated with 40S-80S ribosomal subunits, monosomes, and mRNA/mRNP particles bound to polysomes) (Fig.1). Right, Summary of the measured densities corresponding to the amount of PKCβ2 and RACK1 in fraction_3_ from seven independent experiments. B, Stimulation of hippocampal slices with 0.1 m
m
DHPG for 10 min increases in a comparable manner the amount of RACK1/PKCβ2 in the oligo(dT)-cellulose bound fraction prepared from polysome-bound mRNP complexes (fraction 3).
Fig. 8.
PKCβ2 can phosphorylate a subset of polyA-mRNA-binding proteins. A, PolyA-mRNA/mRNP complexes were prepared from polysome-associated mRNAs and used for an_in vitro_ phosphorylation assay. Addition of33P-γATP resulted in phosphorylation of a number of proteins, indicating the presence of endogenous kinases in this fraction (lane 2); lane 1, addition of a PKC inhibitor peptide (PKC fragment 19–36, 2 μg/ml) reduced endogenous kinase activity only slightly, pointing to additional kinases in this fraction; lane 3, heating the sample for 5 min at 65°C completely abolished the endogenous kinase activities;lane 4, addition of 40 ng PKCβ2 (localization of the phosphorylated kinase is indicated by an arrow) led to phosphorylation of a number of proteins, including a 75 kDa protein that comigrates exactly with Rimb1 (PABP1 and G3BP2a).B, Comparison of in situ phosphorylated mRNPs (first 2 lanes) with mRNPs phosphorylated_in vitro_ by PKCβ2 (lane three). Three endogenous phosphorylated proteins at 75, 30, and 20 kDa (arrows) are also in vitro substrates for PKCβ2 (last lane).
Fig. 9.
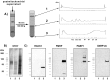
Subcellular localization of RACK1.A, Light micrograph illustrating immunogold-silver staining for RACK1 in a pyramidal neuron in somatosensory cortex. Strong immunolabeling is seen in the neuronal soma and the apical dendrite, and punctate staining in the neuropil suggests synaptic localization of RACK1. Scale bar, 50 μm. B,C, Immunogold-silver labeling for RACK1 (black arrows) in the neuronal cytoplasm (B) and the neuronal nucleus (C). In the cytoplasm the gold-silver particles are localized over ribosomal aggregates (B, white arrows). Scale bars:B, 0.5 μm; C, 1 μm. D, RACK1 immunolabeling in a postsynaptic spine (arrow) close to the postsynaptic density in the spine head. The labeled synapse is surrounded by nonlabeled ones (asterisks) in the neuropil of the somatosensory cortex. Scale bar, 0.5 μm.E, Localization of RACK1 in the cytoplasm of a dendrite. Labeling is associated with cisternae in dendritic cytoplasm. Scale bar, 0.5 μm.
Fig. 10.
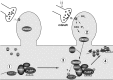
Proposed model for an mGluR-mediated control of postsynaptic protein synthesis. 1, A polyA-mRNA/mRNP complex is transported along microtubules through the dendrite in both anterograde and retrograde directions. The mRNA might be masked by specific mRNPs to prevent premature translation. 2, Synaptic stimulation activates protein kinase Cβ2, via class 1 mGluR.3, Activated PKCβ2 and RACK1 form a complex that translocates and binds to a polyA-mRNA/mRNP complex localized near the stimulated synapse. The presence of activated PKCβ2 within the mRNP complex leads to increased phosphorylation of a subset of mRNPs.4, An alteration in the phosphorylation state of mRNPs may trigger (1) an interruption of mRNP transport that would increase the local amount of mRNAs, (2) a demasking of translational arrested mRNAs, leading to activity-dependent synthesis of specific proteins, or (3) a change in translation efficiency of a subset of postsynaptic localized mRNAs, which could shift the ratio of newly synthesized proteins.
Similar articles
- Proteomic characterization of messenger ribonucleoprotein complexes bound to nontranslated or translated poly(A) mRNAs in the rat cerebral cortex.
Angenstein F, Evans AM, Ling SC, Settlage RE, Ficarro S, Carrero-Martinez FA, Shabanowitz J, Hunt DF, Greenough WT. Angenstein F, et al. J Biol Chem. 2005 Feb 25;280(8):6496-503. doi: 10.1074/jbc.M412742200. Epub 2004 Dec 13. J Biol Chem. 2005. PMID: 15596439 - La-related protein 4 binds poly(A), interacts with the poly(A)-binding protein MLLE domain via a variant PAM2w motif, and can promote mRNA stability.
Yang R, Gaidamakov SA, Xie J, Lee J, Martino L, Kozlov G, Crawford AK, Russo AN, Conte MR, Gehring K, Maraia RJ. Yang R, et al. Mol Cell Biol. 2011 Feb;31(3):542-56. doi: 10.1128/MCB.01162-10. Epub 2010 Nov 22. Mol Cell Biol. 2011. PMID: 21098120 Free PMC article. - RACK1, a protein kinase C anchoring protein, coordinates the binding of activated protein kinase C and select pleckstrin homology domains in vitro.
Rodriguez MM, Ron D, Touhara K, Chen CH, Mochly-Rosen D. Rodriguez MM, et al. Biochemistry. 1999 Oct 19;38(42):13787-94. doi: 10.1021/bi991055k. Biochemistry. 1999. PMID: 10529223 - Principles and properties of eukaryotic mRNPs.
Mitchell SF, Parker R. Mitchell SF, et al. Mol Cell. 2014 May 22;54(4):547-58. doi: 10.1016/j.molcel.2014.04.033. Mol Cell. 2014. PMID: 24856220 Review. - Interaction of protein kinase C with RACK1, a receptor for activated C-kinase: a role in beta protein kinase C mediated signal transduction.
Mochly-Rosen D, Smith BL, Chen CH, Disatnik MH, Ron D. Mochly-Rosen D, et al. Biochem Soc Trans. 1995 Aug;23(3):596-600. doi: 10.1042/bst0230596. Biochem Soc Trans. 1995. PMID: 8566424 Review. No abstract available.
Cited by
- Proteomic analysis of wild-type and mutant huntingtin-associated proteins in mouse brains identifies unique interactions and involvement in protein synthesis.
Culver BP, Savas JN, Park SK, Choi JH, Zheng S, Zeitlin SO, Yates JR 3rd, Tanese N. Culver BP, et al. J Biol Chem. 2012 Jun 22;287(26):21599-614. doi: 10.1074/jbc.M112.359307. Epub 2012 May 3. J Biol Chem. 2012. PMID: 22556411 Free PMC article. - RNG105 deficiency impairs the dendritic localization of mRNAs for Na+/K+ ATPase subunit isoforms and leads to the degeneration of neuronal networks.
Shiina N, Yamaguchi K, Tokunaga M. Shiina N, et al. J Neurosci. 2010 Sep 22;30(38):12816-30. doi: 10.1523/JNEUROSCI.6386-09.2010. J Neurosci. 2010. PMID: 20861386 Free PMC article. - Nervous translation, do you get the message? A review of mRNPs, mRNA-protein interactions and translational control within cells of the nervous system.
Smith R, Rathod RJ, Rajkumar S, Kennedy D. Smith R, et al. Cell Mol Life Sci. 2014 Oct;71(20):3917-37. doi: 10.1007/s00018-014-1660-x. Epub 2014 Jun 22. Cell Mol Life Sci. 2014. PMID: 24952431 Free PMC article. Review. - The La-Related Proteins, a Family with Connections to Cancer.
Stavraka C, Blagden S. Stavraka C, et al. Biomolecules. 2015 Oct 16;5(4):2701-22. doi: 10.3390/biom5042701. Biomolecules. 2015. PMID: 26501340 Free PMC article. Review. - The short conserved region-2 of LARP4 interacts with ribosome-associated RACK1 and promotes translation.
Ranjan A, Mattijssen S, Charlly N, Gallardo IC, Pitman LF, Coleman JC, Conte MR, Maraia RJ. Ranjan A, et al. Nucleic Acids Res. 2025 Jan 24;53(3):gkaf053. doi: 10.1093/nar/gkaf053. Nucleic Acids Res. 2025. PMID: 39898547 Free PMC article.
References
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001;30:489–502. - PubMed
- Angenstein F, Hirschfelder M, Staak S. Activation of metabotropic glutamate receptors increases endogenous protein kinase C substrate phosphorylation in adult hippocampal slices. Brain Res. 1997;745:46–54. - PubMed
- Chang Y-WE, Traugh JA. Phosphorylation of elongation factor 1 and ribosomal protein S6 by multipotential S6 kinase and insulin stimulation of translational elongation. J Biol Chem. 1997;272:28252–28257. - PubMed
- Chicurel ME, Singer RH, Meyer CJ, Ingber DE. Integrin binding and mechanical tension induces movement of mRNA and ribosomes to focal adhesions. Nature. 1998;392:730–733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM 37537/GM/NIGMS NIH HHS/United States
- HD 37175/HD/NICHD NIH HHS/United States
- R01 GM037537/GM/NIGMS NIH HHS/United States
- R37 MH035321/MH/NIMH NIH HHS/United States
- R01 MH035321/MH/NIMH NIH HHS/United States
- MH 35321/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials