The catabolic action of insulin in the brain is mediated by melanocortins - PubMed (original) (raw)
The catabolic action of insulin in the brain is mediated by melanocortins
Stephen C Benoit et al. J Neurosci. 2002.
Abstract
Like leptin, the pancreatic hormone insulin is an important adiposity signal to the brain. We report that the hypothalamic melanocortin system is an important target of the actions of insulin to regulate food intake and body weight. Hypothalamic neurons expressing insulin receptors were found to coexpress the melanocortin precursor molecule pro-opiomelanocortin (POMC), and administration of insulin into the third cerebral ventricle of fasted rats increased expression of POMC mRNA. Finally, a subthreshold dose of the melanocortin antagonist SHU-9119 prevented the reduction in food intake caused by third-ventricular insulin administration. These data suggest that the hypothalamic melanocortin system mediates the anorexic effects of central insulin, as well as of leptin.
Figures
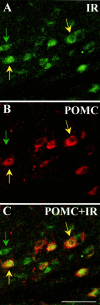
Fig. 1.
Dual-label immunohistochemistry for insulin receptor-β and POMC. The top two panels_are confocal images (5 μm optical section) of ARC neurons. Positive immunoreactivity for the insulin receptor-β is depicted in_green (A), whereas POMC-positive immunoreactivity is depicted in red(B). C, An overlay of the above images. Green arrows point to single-labeled neurons; yellow _arrows_indicate dual-labeled neurons. Scale bar, 50 μm.
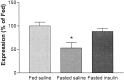
Fig. 2.
Hypothalamic POMC expression. POMC expression after ad libitum feeding (left bar), a 72 hr fast (middle bar), and a 72 hr fast with i3vt infusions of 4 mU of insulin every 12 hr (right bar). Central administration of insulin increased expression of POMC relative to fasting, and the resultant levels were not different from those that occurred during _ad libitum_feeding. *p < 0.05.
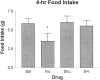
Fig. 3.
Effect of insulin and SHU-9119 on food intake. Mean 4 hr food intake (in grams) after i3vt administration of saline (Sal), insulin (8 mU) (Ins), SHU-9119 (0.1 nmol) (Shu), and SHU-9119 followed by insulin (S+I). SHU-9119, by itself, had no effect on food intake, whereas insulin significantly reduced intake relative to saline. Administration of SHU-9119 before administration of insulin blocked the effect of insulin to reduce food intake (*p < 0.05).
Similar articles
- Hypothalamic, metabolic, and behavioral responses to pharmacological inhibition of CNS melanocortin signaling in rats.
Adage T, Scheurink AJ, de Boer SF, de Vries K, Konsman JP, Kuipers F, Adan RA, Baskin DG, Schwartz MW, van Dijk G. Adage T, et al. J Neurosci. 2001 May 15;21(10):3639-45. doi: 10.1523/JNEUROSCI.21-10-03639.2001. J Neurosci. 2001. PMID: 11331393 Free PMC article. - Effects of streptozotocin-induced diabetes and insulin treatment on the hypothalamic melanocortin system and muscle uncoupling protein 3 expression in rats.
Havel PJ, Hahn TM, Sindelar DK, Baskin DG, Dallman MF, Weigle DS, Schwartz MW. Havel PJ, et al. Diabetes. 2000 Feb;49(2):244-52. doi: 10.2337/diabetes.49.2.244. Diabetes. 2000. PMID: 10868941 - Lean rats with hypothalamic pro-opiomelanocortin overexpression exhibit greater diet-induced obesity and impaired central melanocortin responsiveness.
Li G, Zhang Y, Cheng KY, Scarpace PJ. Li G, et al. Diabetologia. 2007 Jul;50(7):1490-9. doi: 10.1007/s00125-007-0685-1. Epub 2007 May 16. Diabetologia. 2007. PMID: 17505816 - The neuronal histamine H(1) and pro-opiomelanocortin-melanocortin 4 receptors: independent regulation of food intake and energy expenditure.
Yoshimatsu H. Yoshimatsu H. Peptides. 2006 Feb;27(2):326-32. doi: 10.1016/j.peptides.2005.02.028. Epub 2005 Dec 15. Peptides. 2006. PMID: 16343692 Review. - Melanocortins and cardiovascular regulation.
Versteeg DH, Van Bergen P, Adan RA, De Wildt DJ. Versteeg DH, et al. Eur J Pharmacol. 1998 Oct 30;360(1):1-14. doi: 10.1016/s0014-2999(98)00615-3. Eur J Pharmacol. 1998. PMID: 9845266 Review.
Cited by
- β-endorphin differentially contributes to food anticipatory activity in male and female mice undergoing activity-based anorexia.
Daimon CM, Hentges ST. Daimon CM, et al. Physiol Rep. 2021 Mar;9(5):e14788. doi: 10.14814/phy2.14788. Physiol Rep. 2021. PMID: 33661571 Free PMC article. - Reversal of diet-induced obesity increases insulin transport into cerebrospinal fluid and restores sensitivity to the anorexic action of central insulin in male rats.
Begg DP, Mul JD, Liu M, Reedy BM, D'Alessio DA, Seeley RJ, Woods SC. Begg DP, et al. Endocrinology. 2013 Mar;154(3):1047-54. doi: 10.1210/en.2012-1929. Epub 2013 Jan 21. Endocrinology. 2013. PMID: 23337529 Free PMC article. - Obesity: Current and potential pharmacotherapeutics and targets.
Narayanaswami V, Dwoskin LP. Narayanaswami V, et al. Pharmacol Ther. 2017 Feb;170:116-147. doi: 10.1016/j.pharmthera.2016.10.015. Epub 2016 Oct 20. Pharmacol Ther. 2017. PMID: 27773782 Free PMC article. Review. - Direct regulation of the proglucagon gene by insulin, leptin, and cAMP in embryonic versus adult hypothalamic neurons.
Dalvi PS, Erbiceanu FD, Irwin DM, Belsham DD. Dalvi PS, et al. Mol Endocrinol. 2012 Aug;26(8):1339-55. doi: 10.1210/me.2012-1049. Epub 2012 Jun 5. Mol Endocrinol. 2012. PMID: 22669740 Free PMC article. - Appetite Regulation: Hormones, Peptides, and Neurotransmitters and Their Role in Obesity.
Miller GD. Miller GD. Am J Lifestyle Med. 2017 Jun 23;13(6):586-601. doi: 10.1177/1559827617716376. eCollection 2019 Nov-Dec. Am J Lifestyle Med. 2017. PMID: 31662725 Free PMC article. Review.
References
- Barsh GS, Farooqi IS, O'Rahilly S. Genetics of body-weight regulation. Nature. 2000;404:644–651. - PubMed
- Baskin DG, Porte DJ, Guest K, Dorsa DM. Regional concentrations of insulin in the rat brain. Endocr J. 1983;112:898–903. - PubMed
- Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous