Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere - PubMed (original) (raw)
Review
Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere
Charles A Whittaker et al. Mol Biol Cell. 2002 Oct.
Abstract
The von Willebrand A (VWA) domain is a well-studied domain involved in cell adhesion, in extracellular matrix proteins, and in integrin receptors. A number of human diseases arise from mutations in VWA domains. We have analyzed the phylogenetic distribution of this domain and the relationships among approximately 500 proteins containing this domain. Although the majority of VWA-containing proteins are extracellular, the most ancient ones, present in all eukaryotes, are all intracellular proteins involved in functions such as transcription, DNA repair, ribosomal and membrane transport, and the proteasome. A common feature seems to be involvement in multiprotein complexes. Subsequent evolution involved deployment of VWA domains by Metazoa in extracellular proteins involved in cell adhesion such as integrin beta subunits (all Metazoa). Nematodes and chordates separately expanded their complements of extracellular matrix proteins containing VWA domains, whereas plants expanded their intracellular complement. Chordates developed VWA-containing integrin alpha subunits, collagens, and other extracellular matrix proteins (e.g., matrilins, cochlin/vitrin, and von Willebrand factor). Consideration of the known properties of VWA domains in integrins and extracellular matrix proteins allows insights into their involvement in protein-protein interactions and the roles of bound divalent cations and conformational changes. These allow inferences about similar functions in novel situations such as protease regulators (e.g., complement factors and trypsin inhibitors) and intracellular proteins (e.g., helicases, chelatases, and copines).
Figures
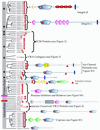
Figure 1
Cell adhesion and extracellular matrix molecules predominate in the diverse collection of human VWA domain-containing proteins. The domain architecture of all human VWA domain-containing proteins is indicated in this figure and the following ones as noted. Paralogous molecules are grouped together in the phylogenetic tree derived from a clustalW alignment. The groups of paralogues or unrooted individual molecules have been shuffled along the vertical axis for clarity of presentation; so there is no information in the root of the tree (vertical gray bar). Molecules with known or likely roles in cell adhesion are marked by black stars in the gray bar on the left-hand side of the figure. Perfect and imperfect MIDAS motifs are indicated by solid and hollow red stars, respectively. All molecules above the purple bar seem to be extracellular; those below the pink bar are intracellular. Signal sequences are designated by a red line and transmembrane segments by a blue bar. Proteins that were assembled or originally characterized in this study are in red, and their sequences are availablein Table S4. See Table S2 to cross-reference molecules in this figure with database identifiers. Figure S2 contains SMART or INTERPRO identifiers for the various domains shown in the diagram (see also
http://web.mit.edu/ccrhq/hyneslab/vwapaper/FigureS2.html
). The diagrams in this and other figures are extracted largely from SMART (
http://smart.embl-heidelberg.de/
) but supplemented in some cases with information from other sources (see text).
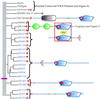
Figure 2
Noncollagenous VWA domain-containing ECM proteins All molecules shown are human except where designated (see text for discussion of orthologues/paralogues). All these proteins are found only in Metazoa and are secreted to the extracellular matrix with the possible exceptions of NG37 and DICE (see text), and most are clearly implicated in cell adhesion. Domains are designated as in Figures 1 and S2. Perfect and imperfect MIDAS motifs are indicated by solid and hollow red stars, respectively.
Figure 3
Sixteen unconventional mammalian collagens contain VWA domains. The figure shows the predicted structures of the eight known unconventional collagens and eight related novel proteins (red), all containing VWA domains. The eight novel proteins have been given letter designations for the purpose of discussion, and their sequences are available in Table S4. Appropriate names should be given after cDNA analysis. On the left is a phylogenetic tree showing only the most confidently clustered groups of paralogues. The vertical gray bar replaces the root of the tree. The VWA domains of these collagens comprise a clade and are more closely related among themselves than with any other VWA domains. They also show familial relationships within the group, shown herein by shading and boxes designating the degree of similarity with percentage bootstrap scores for individual groups shown (yellow ovals). Perfect and imperfect MIDAS motifs are indicated by solid and hollow red stars, respectively. Also marked are all occurrences of RGD motifs (red arrows). Domain structures shown were predicted by SMART except for the collagen motifs (black boxes, each of which represents 20 G-X-Y repeats predicted by Pfam). SMART does not predict VWA 2 or the KU domain in collagen 7α1 but the domains are predicted by Pfam so they were added to the figure. The asterisk (*) indicates that colF is the mouse orthologue used to show C-terminal collagen motifs (see text). The dagger (†) indicates these molecules lack predicted collagen motifs but are included because of close homology of their VWA domains with those of known collagens, as is also true for collagens A–D (see text). aThe human orthologue of chicken collagen 20 is KIAA1510 identified in a full-length cDNA sequencing project (Nagase et al., 2000). bCurrent assemblies a bit ambiguous (see text).
Figure 4
VWA domain-containing proteins common to all eukaryotes are intracellular and found in multiprotein complexes. The five proteins shown are found in all eukaryotes, and we show examples from Metazoa, A. thaliana, and yeast. VWA domains are predicted in orthologues from each of the five. The VWA domain is a particular form of a Rossmann fold and some of the Rossmann folds in orthologues of Ku70/80 and Sec23 do not give predictions for the VWA domain subclass. The VWA domains of TFIIHp44 and Rpn10 are closely related. Both TFIIHp44 and Ku70/80 exhibit helicase activity. A common feature of these proteins is that they are subunits of multiprotein complexes. Given the role of VWA domains in protein–protein interactions, a possible role for the VWA domains in these proteins is in mediating assembly of these complexes. Whatever the role of the VWA domains, these intracellular molecules seem to be the most ancient eukaryotic examples of this protein domain from which other (mostly extracellular) VWA proteins presumably evolved. Molecules that may also be included in this group but have been lost in some taxa include copines (Figure S5). Perfect and imperfect MIDAS motifs are indicated by solid and hollow red stars, respectively. See text for further discussion of these molecules and their relatives.
Figure 5
C. elegans has a large number of novel VWA domain-containing ECM proteins. The domain architecture of all C. elegans VWA domain-containing proteins is indicated. Paralogous molecules are grouped together in the phylogenetic tree derived from a clustalW alignment. The groups of paralogues or unrooted individual molecules have been shuffled along the vertical axis for clarity of presentation; so there is no information in the root of the tree (vertical gray bar). The molecules in blue lack close homologues in all other completed genomes. Note the novel domain associations in many of these proteins. All molecules below the purple bar seem to be extracellular or membrane-associated; the localizations of those below the red bar are unclear. The VWA domains of the C. elegans copines are closely related to the VWA domains of three uncharacterized molecules and these relationships are indicated by a red box. Other relationships are also indicated by boxes and the bootstrap numbers are provided in yellow ovals. Perfect and imperfect MIDAS motifs are indicated by solid and hollow red stars, respectively. See Table S2 to cross-reference molecules in this figure with database identifiers.
Figure 6
A. thaliana has additional intracellular VWA domain proteins. The domain architecture of all_A. thaliana_ VWA domain-containing proteins is indicated. Paralogous molecules are grouped together in the phylogenetic tree derived from a clustalW alignment. The groups of paralogues or unrooted individual molecules have been shuffled along the vertical axis for clarity of presentation; so there is no information in the root of the tree (vertical gray bar). The molecules in blue lack homologues in completed fungal and metazoan genomes. All molecules above the purple bar seem to be intracellular. No information is available for the molecules below the purple bar although one (Q9LSX2) has a predicted signal sequence (indicated by red bar), suggesting that that group of three molecules might be secreted. The VWA domains of the copines are closely related to the VWA domains of the VWA-RING proteins (Q9LVN6 lacks the RING domain). The relationship is indicated by the red box and the number in the yellow oval. Perfect and imperfect MIDAS motifs are indicated by solid and hollow red stars, respectively. See Table S2 to cross-reference molecules in this figure with database identifiers.
Figure 7
Phylogenetic distribution of VWA domain-containing proteins in the organisms with completed genomes is summarized in the diagram. Intracellular proteins are in blue; those with extracellular VWA domains are black. The most ancient VWA domain-containing proteins all seem to be intracellular (see list under early eukaryote). S. cerevisiae and S. pombe have all these genes except copines. With the exception of AAAVWA-ani, metazoan-specific VWA domains are extracellular. Mg chelatases may be of prokaryotic origin with subsequent transfer to plants in chloroplasts. They might also represent the ancestors of AAAVWA-euk, although, at the sequence level, they do not seem closely related. In present-day fungi and plants, it seems that all VWA domain proteins remain intracellular. In contrast, there have been significant expansions in extracellular proteins in Metazoa, a few being widely distributed (e.g., integrin β subunits) and others restricted as in chordates and C. elegans.
Similar articles
- Complement and the multifaceted functions of VWA and integrin I domains.
Springer TA. Springer TA. Structure. 2006 Nov;14(11):1611-6. doi: 10.1016/j.str.2006.10.001. Structure. 2006. PMID: 17098186 Free PMC article. Review. - Evolution of integrin I domains.
Johnson MS, Chouhan BS. Johnson MS, et al. Adv Exp Med Biol. 2014;819:1-19. doi: 10.1007/978-94-017-9153-3_1. Adv Exp Med Biol. 2014. PMID: 25023164 Review. - The cysteine-rich domain of snake venom metalloproteinases is a ligand for von Willebrand factor A domains: role in substrate targeting.
Serrano SM, Kim J, Wang D, Dragulev B, Shannon JD, Mann HH, Veit G, Wagener R, Koch M, Fox JW. Serrano SM, et al. J Biol Chem. 2006 Dec 29;281(52):39746-56. doi: 10.1074/jbc.M604855200. Epub 2006 Oct 13. J Biol Chem. 2006. PMID: 17040908 - Evolution of von Willebrand factor A (VWA) domains.
Tuckwell D. Tuckwell D. Biochem Soc Trans. 1999 Dec;27(6):835-40. doi: 10.1042/bst0270835. Biochem Soc Trans. 1999. PMID: 10830113 Review. No abstract available. - Diversification of von Willebrand Factor A and Chitin-Binding Domains in Pif/BMSPs Among Mollusks.
Shimizu K, Negishi L, Kurumizaka H, Suzuki M. Shimizu K, et al. J Mol Evol. 2024 Aug;92(4):415-431. doi: 10.1007/s00239-024-10180-1. Epub 2024 Jun 12. J Mol Evol. 2024. PMID: 38864871 Free PMC article.
Cited by
- INTS10-INTS13-INTS14 form a functional module of Integrator that binds nucleic acids and the cleavage module.
Sabath K, Stäubli ML, Marti S, Leitner A, Moes M, Jonas S. Sabath K, et al. Nat Commun. 2020 Jul 9;11(1):3422. doi: 10.1038/s41467-020-17232-2. Nat Commun. 2020. PMID: 32647223 Free PMC article. - The MIDASIN and NOTCHLESS genes are essential for female gametophyte development in Arabidopsis thaliana.
Chantha SC, Gray-Mitsumune M, Houde J, Matton DP. Chantha SC, et al. Physiol Mol Biol Plants. 2010 Jan;16(1):3-18. doi: 10.1007/s12298-010-0005-y. Epub 2010 Aug 13. Physiol Mol Biol Plants. 2010. PMID: 23572950 Free PMC article. - HMO-primed bifidobacteria exhibit enhanced ability to adhere to intestinal epithelial cells.
Walsh C, Owens RA, Bottacini F, Lane JA, van Sinderen D, Hickey RM. Walsh C, et al. Front Microbiol. 2023 Dec 15;14:1232173. doi: 10.3389/fmicb.2023.1232173. eCollection 2023. Front Microbiol. 2023. PMID: 38163079 Free PMC article. - The Goblet Cell Protein Clca1 (Alias mClca3 or Gob-5) Is Not Required for Intestinal Mucus Synthesis, Structure and Barrier Function in Naive or DSS-Challenged Mice.
Erickson NA, Nyström EE, Mundhenk L, Arike L, Glauben R, Heimesaat MM, Fischer A, Bereswill S, Birchenough GM, Gruber AD, Johansson ME. Erickson NA, et al. PLoS One. 2015 Jul 10;10(7):e0131991. doi: 10.1371/journal.pone.0131991. eCollection 2015. PLoS One. 2015. PMID: 26162072 Free PMC article. - The alpha-kinase family: an exceptional branch on the protein kinase tree.
Middelbeek J, Clark K, Venselaar H, Huynen MA, van Leeuwen FN. Middelbeek J, et al. Cell Mol Life Sci. 2010 Mar;67(6):875-90. doi: 10.1007/s00018-009-0215-z. Epub 2009 Dec 12. Cell Mol Life Sci. 2010. PMID: 20012461 Free PMC article. Review.
References
- Adams MD, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. - PubMed
- Anand G, Yin X, Shahidi AK, Grove L, Prochownik EV. Novel regulation of the helix-loop-helix protein Id1 by S5a, a subunit of the 26 S proteasome. J Biol Chem. 1997;272:19140–19151. - PubMed
- Anantharaman V, Aravind L. Cachea signaling domain common to animal Ca(2+)-channel subunits, and a class of prokaryotic chemotaxis receptors. Trends Biochem Sci. 2000;25:535–537. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases