ARF1.GTP, tyrosine-based signals, and phosphatidylinositol 4,5-bisphosphate constitute a minimal machinery to recruit the AP-1 clathrin adaptor to membranes - PubMed (original) (raw)
ARF1.GTP, tyrosine-based signals, and phosphatidylinositol 4,5-bisphosphate constitute a minimal machinery to recruit the AP-1 clathrin adaptor to membranes
Pascal Crottet et al. Mol Biol Cell. 2002 Oct.
Abstract
At the trans-Golgi network, clathrin coats containing AP-1 adaptor complexes are formed in an ARF1-dependent manner, generating vesicles transporting cargo proteins to endosomes. The mechanism of site-specific targeting of AP-1 and the role of cargo are poorly understood. We have developed an in vitro assay to study the recruitment of purified AP-1 adaptors to chemically defined liposomes presenting peptides corresponding to tyrosine-based sorting motifs. AP-1 recruitment was found to be dependent on myristoylated ARF1, GTP or nonhydrolyzable GTP-analogs, tyrosine signals, and small amounts of phosphoinositides, most prominently phosphatidylinositol 4,5-bisphosphate, in the absence of any additional cytosolic or membrane bound proteins. AP-1 from cytosol could be recruited to a tyrosine signal independently of the lipid composition, but the rate of recruitment was increased by phosphatidylinositol 4,5-bisphosphate. The results thus indicate that cargo proteins are involved in coat recruitment and that the local lipid composition contributes to specifying the site of vesicle formation.
Figures
Figure 1
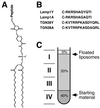
Peptidoliposomes to assay AP-1 recruitment in vitro. The maleimide derivative of PE MMCC-DHPE was used to couple synthetic peptides via an N-terminal cysteine to a lipid (A). The peptides used correspond to the cytoplasmic domain of Lamp1 (B, Lamp1Y) or the segment of TGN38 that has previously been shown to contain the functional tyrosine motif (Boll et al., 1996). Lamp1A and TGN38A are the control peptides with the critical tyrosine mutated to alanine. After incubation of peptidoliposomes with AP-1 and with or without ARF1, they were floated from the bottom of a sucrose step gradient (C). Four fractions were collected as indicated, with fraction I containing the floated liposomes with bound proteins and fraction IV including the loading zone with unbound proteins.
Figure 2
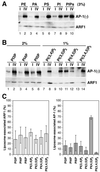
AP-1 recruitment to peptidoliposomes is signal-, ARF1- and lipid-dependent. (A) Peptidoliposomes made of 100% PC or 50% PC/50% soybean lipids and presenting Lamp1Y or Lamp1A peptides were incubated with a mixed adaptor preparation and with or without myristoylated ARF1 and GMP-PNP. After flotation on a sucrose step gradient, four fractions (I–IV, as shown in Figure 1C) were collected from the top and analyzed by immunoblotting for γ-adaptin or ARF1. (B) The same experiments were performed using peptidoliposomes made of 50% PC/50% soybean lipids and presenting TGN38Y or TGN38A peptides.
Figure 3
Lipid requirement for AP-1 recruitment to peptidoliposomes. (A) Three percent of the indicated lipid was incorporated into PC peptidoliposomes exposing Lamp1Y. After incubation with a mixed adaptor preparation and with myristoylated ARF1·GMP-PNP, fractions I and IV of a flotation gradient were analyzed by immunoblotting. PIPs indicates a commercial mixture of phosphoinositides. (B) Two percent of PI-monophosphates and 1% of PI-bis- and trisphosphates were incorporated into PC peptidoliposomes exposing Lamp1Y and analyzed as in A. (C) The recruitment of AP-1 and ARF1 to liposomes containing different phosphoinositides (2% of PI-monophosphates and 1% of PI-bis- and trisphosphates) were densitometrically quantified. The amount recovered in fraction I is expressed in percent of the total in fractions I plus IV. The average and SDs of at least three experiments, including those shown in B, are presented.
Figure 4
Nucleotide dependence of AP-1 recruitment to peptidoliposomes. The indicated nucleotide was incubated with myristoylated or nonmyristoylated ARF1 and peptidoliposomes containing 3% of mixed inositides and exposing Lamp1Y. The analysis was performed as in Figure 3.
Figure 5
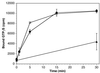
Nucleotide exchange on ARF1. Myristoylated ARF1 was incubated at 37°C with [35S]GTPγS and either buffer only (▴), PC liposomes (●), or PC with 10% mixed phosphoinositides (□). At the indicated times, samples were quickly filtered through a nitrocellulose filter. After washing, the radioactivity on the filter, corresponding to GTPγS bound to ARF1, was counted.
Figure 6
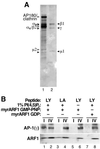
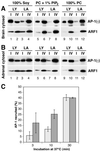
Recruitment of pure AP-1 to peptidoliposomes. (A) Aliquots of the mixed adaptor preparation (lane 1) and of hydroxyapatite-purified AP-1 (lane 2) containing the same amount of AP-1 (as judged by immunoblot analysis) were separated by SDS-gel electrophoresis and stained with silver. AP-1 subunits β1, γ, and μ1 are indicated by filled arrowheads, whereas AP180 and AP-2 subunits αa, αc, β2, and μ2 are indicated by open arrowheads. (B) AP-1 recruitment assays were performed using liposomes made of PC with or without 1% PI(4,5)P2 and exposing Lamp1Y (LY) or Lamp1A (LA) peptides in the presence of myristoylated ARF1 loaded with GMP-PNP or GDP. The analysis was performed as in Figure 3.
Figure 7
Recruitment of AP-1 from cytosol. AP-1 recruitment assays were performed using brain cytosol (A) or adrenal gland cytosol (B), and peptidoliposomes made of soybean lipids (lanes 1–4), PC with 1% PI(4,5)P2 (lanes 5–8), or pure PC (lanes 9–12), exposing Lamp1Y (LY) or Lamp1A (LA) peptides. Cytosol supplemented with purified ARF1 and GMP-PNP was incubated with the peptidoliposomes for 30 min at 37°C before separation by gradient centrifugation. (C) To determine the kinetics, AP-1 recruitment assays were performed using brain cytosol and liposomes exposing Lamp1Y peptides prepared of either PC alone (white bars) or PC containing 1% PI(4,5)P2 (dark bars) at different incubation times (average and SD of 3 determinations).
Similar articles
- Cargo-sorting signals promote polymerization of adaptor protein-1 in an Arf-1.GTP-independent manner.
Lee I, Drake MT, Traub LM, Kornfeld S. Lee I, et al. Arch Biochem Biophys. 2008 Nov 1;479(1):63-8. doi: 10.1016/j.abb.2008.08.009. Epub 2008 Aug 20. Arch Biochem Biophys. 2008. PMID: 18762162 Free PMC article. - Recruitment of coat proteins to peptidoliposomes.
Suri G, Spiess M, Crottet P. Suri G, et al. Methods Mol Biol. 2008;457:227-39. doi: 10.1007/978-1-59745-261-8_17. Methods Mol Biol. 2008. PMID: 19066031 - The assembly of AP-3 adaptor complex-containing clathrin-coated vesicles on synthetic liposomes.
Drake MT, Zhu Y, Kornfeld S. Drake MT, et al. Mol Biol Cell. 2000 Nov;11(11):3723-36. doi: 10.1091/mbc.11.11.3723. Mol Biol Cell. 2000. PMID: 11071902 Free PMC article. - Cargo adaptors: structures illuminate mechanisms regulating vesicle biogenesis.
Paczkowski JE, Richardson BC, Fromme JC. Paczkowski JE, et al. Trends Cell Biol. 2015 Jul;25(7):408-16. doi: 10.1016/j.tcb.2015.02.005. Epub 2015 Mar 17. Trends Cell Biol. 2015. PMID: 25795254 Free PMC article. Review. - Conserved functions of membrane active GTPases in coated vesicle formation.
Pucadyil TJ, Schmid SL. Pucadyil TJ, et al. Science. 2009 Sep 4;325(5945):1217-20. doi: 10.1126/science.1171004. Science. 2009. PMID: 19729648 Free PMC article. Review.
Cited by
- Functional and genomic changes in the mouse ocular motor system in response to light deprivation from birth.
McMullen CA, Andrade FH, Stahl JS. McMullen CA, et al. J Neurosci. 2004 Jan 7;24(1):161-9. doi: 10.1523/JNEUROSCI.3234-03.2004. J Neurosci. 2004. PMID: 14715949 Free PMC article. - Glucose starvation inhibits autophagy via vacuolar hydrolysis and induces plasma membrane internalization by down-regulating recycling.
Lang MJ, Martinez-Marquez JY, Prosser DC, Ganser LR, Buelto D, Wendland B, Duncan MC. Lang MJ, et al. J Biol Chem. 2014 Jun 13;289(24):16736-47. doi: 10.1074/jbc.M113.525782. Epub 2014 Apr 21. J Biol Chem. 2014. PMID: 24753258 Free PMC article. - Human immunodeficiency virus type 1 Nef: adapting to intracellular trafficking pathways.
Roeth JF, Collins KL. Roeth JF, et al. Microbiol Mol Biol Rev. 2006 Jun;70(2):548-63. doi: 10.1128/MMBR.00042-05. Microbiol Mol Biol Rev. 2006. PMID: 16760313 Free PMC article. Review. - Structural basis for recruitment and activation of the AP-1 clathrin adaptor complex by Arf1.
Ren X, Farías GG, Canagarajah BJ, Bonifacino JS, Hurley JH. Ren X, et al. Cell. 2013 Feb 14;152(4):755-67. doi: 10.1016/j.cell.2012.12.042. Cell. 2013. PMID: 23415225 Free PMC article. - The synaptojanin-like protein Inp53/Sjl3 functions with clathrin in a yeast TGN-to-endosome pathway distinct from the GGA protein-dependent pathway.
Ha SA, Torabinejad J, DeWald DB, Wenk MR, Lucast L, De Camilli P, Newitt RA, Aebersold R, Nothwehr SF. Ha SA, et al. Mol Biol Cell. 2003 Apr;14(4):1319-33. doi: 10.1091/mbc.e02-10-0686. Mol Biol Cell. 2003. PMID: 12686590 Free PMC article.
References
- Antonny B, Beraud-Dufour S, Chardin P, Chabre M. N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry. 1997;36:4675–4684. - PubMed
- Antonny B, Madden D, Hamamoto S, Orci L, Schekman R. Dynamics of the COPII coat with GTP and stable analogues. Nat Cell Biol. 2001;3:531–537. - PubMed
- Austin C, Hinners I, Tooze SA. Direct and GTP-dependent interaction of ADP-ribosylation factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J Biol Chem. 2000;275:21862–21869. - PubMed
- Beck KA, Keen JH. Interaction of phosphoinositide cycle intermediates with the plasma membrane-associated clathrin assembly protein AP-2. J Biol Chem. 1991;266:4442–4447. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases