Transneuronal tracing of diverse CNS circuits by Cre-mediated induction of wheat germ agglutinin in transgenic mice - PubMed (original) (raw)
Transneuronal tracing of diverse CNS circuits by Cre-mediated induction of wheat germ agglutinin in transgenic mice
Joao M Braz et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Systems neuroscience addresses the complex circuits made by populations of neurons in the CNS and the cooperative function of these neurons. Improved approaches to the neuroanatomical analysis of CNS circuits are thus of great interest. In fact, significant advances in tract-tracing methods have recently been made by using transgenic mice that express transneuronal lectin tracers under the control of neuron-specific promoters. The utility of those animals, however, is limited to the CNS circuit influenced by the particular promoter. Here, we describe a new transgenic mouse that can be used for transneuronal tracing analysis of circuits in any region of the brain or spinal cord. The transgene in these mice results in expression of LacZ in neurons throughout the CNS. Excision of the LacZ gene by Cre-mediated recombination initiates expression of the lectin, wheat germ agglutinin (WGA). To illustrate the diverse uses of these ZW (LacZ-WGA) mice, we triggered WGA expression either by crossing the mice with two Cre-expressing transgenic mouse lines or by microinjecting a Cre-expressing adeno-associated virus into the cerebellum or cerebral cortex. Both approaches resulted in extensive WGA expression in the cell bodies and dendrites of neurons in which the recombination event occurred, as well as anterograde and transneuronal transport of the lectin to second and third order neurons. Because the lectin can be induced in developing and adult animals, and in all regions of the brain and spinal cord, these ZW may prove extremely valuable for numerous studies of CNS circuit analysis.
Figures
Fig 1.
pCZW construct used to generate ZW transgenic mice. Both the LacZ (β-galactosidase-neomycin resistance fusion protein) and WGA expression are under the control of the ubiquitous chicken β-actin promoter-cytomegalovirus enhancer. Under normal conditions, only the LacZ is expressed. After Cre recombination, the loxP-flanked LacZ is excised, and the WGA is transcribed.
Fig 2.
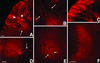
Expression of the ZW transgene in adult transgenic mice. (A) This photomontage of a sagittal section of the brain of a 2-wk-old ZW mouse shows β-galactosidase activity throughout the CNS, indicating that there is extensive and widespread expression of the transgene. (B) Based on the double labeling (yellow) of NeuN-positive (green) and β-galactosidase-positive neurons (red), we estimate that about half of the total neuron population expresses the ZW transgene. This particular image was taken from a section through the striatum. (C) The ZW transgene is expressed in a mosaic fashion. Although the labeling in Purkinje cells was the most prominent, we observed extensive, albeit light labeling of all cell layers of the cerebellum. (D) After Cre recombination in an ZW/L7-Cre double transgenic mouse, Purkinje cells no longer express the LacZ transgene and thus the Purkinje cell layer appears white. Shown are granule cell (gc), molecular (m), and Purkinje cell layers (P). (Bar = 1.0 mm in A and 75 μm in B_–_D.)
Fig 3.
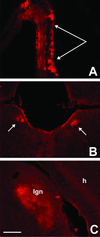
WGA expression and transneuronal transport in cerebellar pathways of ZW/L7-Cre double transgenic mice. After Cre recombination, the WGA immunostaining (red) localizes extensively in Purkinje cells (A and C). The lectin is uniformly distributed in Purkinje cell somata, dendrites, and axons (arrow in D), and axonal arbors are readily detected in the deep cerebellar and vestibular nuclei (arrowheads and arrow in A). The tracer is axonally transported and transneuronally transferred to second order neurons in the deep cerebellar nuclei (B) and to third order neurons (arrows) in the red nucleus (E). (F) Transneuronal label in the ventrolateral nucleus of the thalamus appeared to be restricted to terminals. (Bar = 450 μm in A and 150 μm in B_–_F.)
Fig 4.
WGA expression in vision circuits of α-Cre/ZW double transgenic mice. α-Cre mice express the Cre recombinase only in retinal progenitor cells that give rise to all cell types of the mature neuroretina. (A) After crossing with ZW mice, we found WGA immunoreactivity in cell bodies and dendrites through all retinal layers, with a concentration in the peripheral retina. Arrows in A point to photoreceptor layer. The WGA protein was anterogradely transferred from retinal ganglion cells to the pretectal nuclei (B) and to neurons in the dorsal lateral geniculate nucleus (C). We did not find labeled cortical neurons in these animals. (Bar = 200 μm in A and 100 μm in B and C.)
Fig 5.
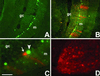
WGA expression and transneuronal transport after AAV-mediated Cre-GFP delivery to the cerebellar cortex of an adult ZW mouse. (A) GFP fluorescence (resulting from expression of the Cre-GFP fusion protein) identifies the location of the AAV injection, which was made 7 days before, in this 6-wk-old mouse. The Cre recombinase was mainly found in the nuclei of infected cells. (B) An adjacent section reveals that Cre recombination triggered WGA expression (red) in several cerebellar cells. (C) The WGA (red) was transneuronally transferred to noninfected Purkinje cells (arrow), i.e., which contained the βgeo transgene. Some Cre-expressing Purkinje cells (green, arrowhead) are not WGA positive, due to the mosaicism of the transgene expression. (D) As observed in the ZW/L7-Cre double transgenic mice, AAV induced-Cre recombination in Purkinje cells was followed by transneuronal transport of the WGA to neurons in the deep cerebellar nuclei. (Bar = 200 μm in A_–_D.)
Fig 6.
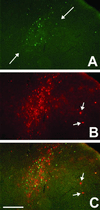
WGA expression and transneuronal transport after AAV-mediated Cre-GFP delivery to the visual cortex of an adult ZW mouse. (A) GFP fluorescence shows that AAV-infected neurons were concentrated in the middle laminae of the cortex. (B) The WGA immunoreactivity (red) overlaps extensively with the GFP labeling but also extends to more superficial and deep cortical layers, in a columnar pattern. (C) The merged image shows that there is also transneuronal transport of the lectin into cortical neurons that are likely located in an adjacent column (arrow). (Bar = 150 μm in A_–_C.)
Similar articles
- Evaluation of WGA-Cre-dependent topological transgene expression in the rodent brain.
Libbrecht S, Van den Haute C, Malinouskaya L, Gijsbers R, Baekelandt V. Libbrecht S, et al. Brain Struct Funct. 2017 Mar;222(2):717-733. doi: 10.1007/s00429-016-1241-x. Epub 2016 Jun 3. Brain Struct Funct. 2017. PMID: 27259586 - Triggering genetically-expressed transneuronal tracers by peripheral axotomy reveals convergent and segregated sensory neuron-spinal cord connectivity.
Bráz JM, Basbaum AI. Bráz JM, et al. Neuroscience. 2009 Nov 10;163(4):1220-32. doi: 10.1016/j.neuroscience.2009.07.051. Epub 2009 Jul 30. Neuroscience. 2009. PMID: 19647044 Free PMC article. - Generation of Oxtr cDNA(HA)-Ires-Cre Mice for Gene Expression in an Oxytocin Receptor Specific Manner.
Hidema S, Fukuda T, Hiraoka Y, Mizukami H, Hayashi R, Otsuka A, Suzuki S, Miyazaki S, Nishimori K. Hidema S, et al. J Cell Biochem. 2016 May;117(5):1099-111. doi: 10.1002/jcb.25393. Epub 2015 Nov 24. J Cell Biochem. 2016. PMID: 26442453 - A fourth generation of neuroanatomical tracing techniques: exploiting the offspring of genetic engineering.
Wouterlood FG, Bloem B, Mansvelder HD, Luchicchi A, Deisseroth K. Wouterlood FG, et al. J Neurosci Methods. 2014 Sep 30;235:331-48. doi: 10.1016/j.jneumeth.2014.07.021. Epub 2014 Aug 11. J Neurosci Methods. 2014. PMID: 25107853 Review. - Cre Activated and Inactivated Recombinant Adeno-Associated Viral Vectors for Neuronal Anatomical Tracing or Activity Manipulation.
Saunders A, Sabatini BL. Saunders A, et al. Curr Protoc Neurosci. 2015 Jul 1;72:1.24.1-1.24.15. doi: 10.1002/0471142301.ns0124s72. Curr Protoc Neurosci. 2015. PMID: 26131660 Free PMC article. Review.
Cited by
- Inhibitory Kcnip2 neurons of the spinal dorsal horn control behavioral sensitivity to environmental cold.
Albisetti GW, Ganley RP, Pietrafesa F, Werynska K, Magalhaes de Sousa M, Sipione R, Scheurer L, Bösl MR, Pelczar P, Wildner H, Zeilhofer HU. Albisetti GW, et al. Neuron. 2023 Jan 4;111(1):92-105.e5. doi: 10.1016/j.neuron.2022.10.008. Epub 2022 Nov 1. Neuron. 2023. PMID: 36323322 Free PMC article. - Transgenic targeting of recombinant rabies virus reveals monosynaptic connectivity of specific neurons.
Weible AP, Schwarcz L, Wickersham IR, Deblander L, Wu H, Callaway EM, Seung HS, Kentros CG. Weible AP, et al. J Neurosci. 2010 Dec 8;30(49):16509-13. doi: 10.1523/JNEUROSCI.2442-10.2010. J Neurosci. 2010. PMID: 21147990 Free PMC article. - Canine Adenovirus 2: A Natural Choice for Brain Circuit Dissection.
Lavoie A, Liu BH. Lavoie A, et al. Front Mol Neurosci. 2020 Feb 27;13:9. doi: 10.3389/fnmol.2020.00009. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32174812 Free PMC article. Review. - "Small axonless neurons": postnatally generated neocortical interneurons with delayed functional maturation.
Le Magueresse C, Alfonso J, Khodosevich K, Arroyo Martín AA, Bark C, Monyer H. Le Magueresse C, et al. J Neurosci. 2011 Nov 16;31(46):16731-47. doi: 10.1523/JNEUROSCI.4273-11.2011. J Neurosci. 2011. PMID: 22090500 Free PMC article.
References
- Kobbert C., Apps, R., Bechmann, I., Lanciego, J. L., Mey, J. & Thanos, S. (2000) Prog. Neurobiol. 62, 327-351. - PubMed
- Card J. P. & Enquist, L. W. (1995) Crit. Rev. Neurobiol. 9, 137-162. - PubMed
- Loewy A. D. (1998) Neurosci. Biobehav. Rev. 22, 679-684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 21445/PHS HHS/United States
- R01 NS014627/NS/NINDS NIH HHS/United States
- DA 08377/DA/NIDA NIH HHS/United States
- R37 NS014627/NS/NINDS NIH HHS/United States
- NS 14627/NS/NINDS NIH HHS/United States
- P01NS 16033/NS/NINDS NIH HHS/United States
- P01 NS016033/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases