Unfolded cholera toxin is transferred to the ER membrane and released from protein disulfide isomerase upon oxidation by Ero1 - PubMed (original) (raw)
Unfolded cholera toxin is transferred to the ER membrane and released from protein disulfide isomerase upon oxidation by Ero1
Billy Tsai et al. J Cell Biol. 2002.
Abstract
The toxic effect of cholera toxin (CT) on target cells is caused by its A1 chain. This polypeptide is released from the holotoxin and unfolded in the lumen of the ER by the action of protein disulfide isomerase (PDI), before being retrotranslocated into the cytosol. The polypeptide is initially unfolded by binding to the reduced form of PDI. We show that upon oxidation of the COOH-terminal disulfide bond in PDI by the enzyme Ero1, the A1 chain is released. Both yeast Ero1 and the mammalian Ero1alpha isoform are active in this reaction. Ero1 has a preference for the PDI-toxin complex. We further show that the complex is transferred to a protein at the lumenal side of the ER membrane, where the unfolded toxin is released from PDI by the action of Ero1. Taken together, our results identify Ero1 as the enzyme mediating the release of unfolded CT from PDI and characterize an additional step in retrotranslocation of the toxin.
Figures
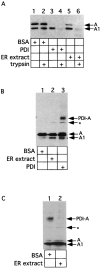
Figure 1.
An ER activity induces release of the A1 chain from PDI. (A) Isolated A subunit (70 nM) was incubated with either BSA (3 μM), purified mammalian PDI (3 μM), or ER extract (3 mg/ml) in GSH (1 mM). Where indicated, trypsin (100 μg/ml) was added. Samples were analyzed by nonreducing SDS-PAGE and immunoblotting with a CT antibody. The positions of the proteolytically cleaved A subunit (containing A1 and A2 chains linked by a disulfide bond) and of the A1 chain are indicated. (B) Isolated A subunit (70 nM) was incubated with either BSA (3 μM), ER extract (3 mg/ml) or purified PDI (3 μM) in GSH (1 mM). A carbodiimide crosslinker (EDAC) was then added. Samples were analyzed as in (A). PDI-A represents a crosslinked product between PDI and the A subunit. * indicates an unidentified band. (C) Isolated A subunit (70 nM) was first incubated with purified PDI (3 μM) in GSH (1 mM), followed by addition of either BSA (3 μM) or ER extract (3 mg/ml). Samples were analyzed as in (B).
Figure 2.
Ero1 induces the release of the A1 peptide from reduced PDI. (A) Protein from five equivalents of dog pancreatic microsomes were treated with or without endo H and analyzed by SDS-PAGE followed by immunoblotting with antibodies to Ero1α/β (lanes 1 and 2), Ero1α (lanes 3 and 4), or Ero1β (lanes 5 and 6). Purified mammalian Ero1α (1 μg), analyzed in the Coomassie gel (lane 7), was tested with antibodies to Ero1α/β, Ero1α, or Ero1β (lanes 8–10). x represents an uncharacterized glycosylated form of Ero1α. (B) ER extract or Ero1- depleted extract (depleted by incubation with either antibody 193 or 194) were analyzed by immunoblotting with antibodies to Ero1α/β (antibody 194, lanes 1–3), Ero1α (lanes 4–6), or Ero1β (lanes 7–9). Lanes 10–14 show the results of release assays. Isolated A subunit (70 nM) and PDI (3 μM) were incubated in GSH (1 mM) followed by addition of BSA (2 mg/ml), ER extract (2 mg/ml), Ero1-depleted extract (depleted by antibody 193 or 194), or GSSG (30 mM; lanes 10–14). The samples were analyzed as in Fig. 1 B. (C) Purified mammalian Ero1α (0.3 μM or 3 μM) was added to the PDI–toxin complex and analyzed as in Fig. 1 B (lanes 1–3). Purified yeast Ero1 (Coomassie gel, lane 4) was added (0.3 μM or 1.5 μM) to the toxin-PDI complex and the samples were analyzed as in Fig. 1 B (lanes 5–7).
Figure 3.
Release activity is present in Ero1α-containing fractions. (A) The protein in an ER extract was bound to a Q-Sepharose column and eluted with a continuous salt gradient. Fractions were analyzed for its Ero1 content by SDS-PAGE and immunoblotting with antibodies that either recognize both Ero1 isoforms (top), Ero1α only (middle), or Ero1β only (bottom). (B) The fractions were tested for their ability to induce toxin release, as described in Fig. 1 B.
Figure 4.
Ero1 oxidizes the COOH-terminal disulfide bond in PDI and acts preferentially on the toxin–PDI complex. (A) Isolated A subunit (70 nM) and purified mammalian PDI (3 μM) were incubated in the presence of DTT (100 mM), GSSG (100 mM), or GSH (1 mM). Where indicated, purified mammalian Ero1α (0.3 μM or 3 μM) was added. 1/10 of the sample was TCA precipitated, washed with acetone, and treated with maleimide-PEG5000. The samples were subsequently analyzed by nonreducing SDS-PAGE followed by immunoblotting with an antibody against PDI. Arrows point to the number of cysteines in PDI modified with maleimide-PEG5000 (-SH-MP). (B) As in A except that purified yeast Ero1(0.3 μM or 1.5 μM) was used. (C) As in B except that 30 μM yeast Ero1 was added. (D) As in C except that 3 μM yeast mutant PDICxxC-AxxA or PDIAxxA-CxxC were used. (E) As in B except 70 nM or 3 μM of isolated A subunit were incubated with PDI (3 μM).
Figure 5.
PDI-unfolded A1 peptide is transferred to the ER membrane. (A) Isolated A subunit (70 nM) was incubated in the presence of GSH (1 mM) with ER extract, proteoliposomes, or ER extract followed by proteoliposomes (lanes 1–6). After sedimentation, the proteoliposome-bound toxin in the pellet fraction and the unbound fraction in the supernatant were analyzed by nonreducing SDS-PAGE followed by immunoblotting. Controls were performed with either native microsomes (lanes 9 and 10), liposomes lacking proteins (lanes 7 and 8), or proteoliposomes generated from a proteinase K-treated detergent extract (lanes 15 and 16). (B) Isolated A subunit (70 nM) was incubated with either BSA, ER extract, or 6M urea (lanes 1–6). Where indicated, trypsin (100 μg/ml) was added. Samples were analyzed as in A. In lanes 7–10, isolated A subunit (70 nM) was incubated with either ER extract or 6M urea (followed by addition of proteoliposomes. Samples were analyzed as in A. (C) In lanes 1–3, ER extract or PDI-depleted extract were analyzed by immunoblotting with PDI antibodies. In lanes 4–7, isolated A subunit (70 nM) was incubated with these extracts, followed by the addition of proteoliposomes and the samples were separated into pellet and supernatant fractions. In lanes 8–11, isolated A subunit (70 nM) was incubated with purified PDI or BSA followed by addition of proteoliposomes. (D) As in Fig. 1 C, except BSA, wild-type mammalian proteoliposomes, wild-type yeast proteoliposomes, or yeast mutant ero1–1 proteoliposomes were added to the preformed PDI–toxin complex.
Similar articles
- FAD oxidizes the ERO1-PDI electron transfer chain: the role of membrane integrity.
Papp E, Nardai G, Mandl J, Bánhegyi G, Csermely P. Papp E, et al. Biochem Biophys Res Commun. 2005 Dec 16;338(2):938-45. doi: 10.1016/j.bbrc.2005.10.027. Epub 2005 Oct 17. Biochem Biophys Res Commun. 2005. PMID: 16246310 - Protein disulfide isomerase-like proteins play opposing roles during retrotranslocation.
Forster ML, Sivick K, Park YN, Arvan P, Lencer WI, Tsai B. Forster ML, et al. J Cell Biol. 2006 Jun 19;173(6):853-9. doi: 10.1083/jcb.200602046. J Cell Biol. 2006. PMID: 16785320 Free PMC article. - Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin.
Tsai B, Rodighiero C, Lencer WI, Rapoport TA. Tsai B, et al. Cell. 2001 Mar 23;104(6):937-48. doi: 10.1016/s0092-8674(01)00289-6. Cell. 2001. PMID: 11290330 - Protein disulfide isomerase: the structure of oxidative folding.
Gruber CW, Cemazar M, Heras B, Martin JL, Craik DJ. Gruber CW, et al. Trends Biochem Sci. 2006 Aug;31(8):455-64. doi: 10.1016/j.tibs.2006.06.001. Epub 2006 Jul 11. Trends Biochem Sci. 2006. PMID: 16815710 Review. - Molecular mechanisms regulating oxidative activity of the Ero1 family in the endoplasmic reticulum.
Tavender TJ, Bulleid NJ. Tavender TJ, et al. Antioxid Redox Signal. 2010 Oct;13(8):1177-87. doi: 10.1089/ars.2010.3230. Antioxid Redox Signal. 2010. PMID: 20486761 Review.
Cited by
- Insights on the trafficking and retro-translocation of glycosphingolipid-binding bacterial toxins.
Cho JA, Chinnapen DJ, Aamar E, te Welscher YM, Lencer WI, Massol R. Cho JA, et al. Front Cell Infect Microbiol. 2012 Apr 11;2:51. doi: 10.3389/fcimb.2012.00051. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919642 Free PMC article. Review. - An elusive adenylate cyclase complicit in cholera is exposed.
Randak CO. Randak CO. J Biol Chem. 2018 Aug 17;293(33):12960-12961. doi: 10.1074/jbc.H118.004669. J Biol Chem. 2018. PMID: 30120153 Free PMC article. Review. - Cholera toxin - a foe & a friend.
Sanchez J, Holmgren J. Sanchez J, et al. Indian J Med Res. 2011 Feb;133(2):153-63. Indian J Med Res. 2011. PMID: 21415489 Free PMC article. Review. - Generating an unfoldase from thioredoxin-like domains.
Forster ML, Mahn JJ, Tsai B. Forster ML, et al. J Biol Chem. 2009 May 8;284(19):13045-56. doi: 10.1074/jbc.M808352200. Epub 2009 Mar 16. J Biol Chem. 2009. PMID: 19289469 Free PMC article. - How viruses use the endoplasmic reticulum for entry, replication, and assembly.
Inoue T, Tsai B. Inoue T, et al. Cold Spring Harb Perspect Biol. 2013 Jan 1;5(1):a013250. doi: 10.1101/cshperspect.a013250. Cold Spring Harb Perspect Biol. 2013. PMID: 23284050 Free PMC article. Review.
References
- Cabibbo, A., M. Pagani, M. Fabbri, M. Rocchi, M.R. Farmery, N.J. Bulleid, and R. Sitia. 2000. ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J. Biol. Chem. 275:4827–4833. - PubMed
- Frand, A.R., and C.A. Kaiser. 1998. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol. Cell. 1:161–170. - PubMed
- Frand, A.R., and C.A. Kaiser. 1999. Ero1p oxidizes protein disulfide isomerase in a pathway for disulfide bond formation in the endoplasmic reticulum. Mol. Cell. 4:469–477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases