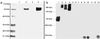A previously undescribed chemical link between smoking and metabolic disease - PubMed (original) (raw)
A previously undescribed chemical link between smoking and metabolic disease
Tobin J Dickerson et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Over the past 20 years, protein glycation has been implicated in a variety of pathological states. Although smoking also can contribute to many of these diseases, the precise mechanism by which this occurs is not known. Previously, we have demonstrated that nornicotine, a constituent of tobacco and metabolite of nicotine, can catalyze aldol reactions under aqueous conditions. This finding has caused us to question whether this reaction has physiological consequences. We now report that nornicotine causes aberrant protein glycation and catalyzes the covalent modification of certain prescription drugs such as the commonly used steroid, prednisone. Furthermore, we show that the plasma of smokers as compared with nonsmokers contains higher concentrations of nornicotine-modified proteins, suggesting an unrecognized pathway for the development of the pathology of tobacco abuse.
Figures
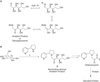
Fig 1.
(a) Amadori product derived from the reaction of glucose and a primary amine. (b) Formation of nornicotine-based Amadori product 2 from glucose and nornicotine 1. This intermediate can undergo further chemical reaction including oxidation to a 1,2-dicarbonyl-containing compound, a critical intermediate found in protein glycation.
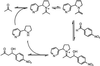
Fig 2.
Covalent catalysis of the aldol reaction by nornicotine via an enamine intermediate.
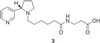
Fig 3.
Hapten 3 used to elicit anti-nicotine mAbs.
Fig 4.
Chemical modification of proteins with nornicotine. SDS/PAGE and Western blot with mAb NIC6C12 of samples are shown. (a) BSA (lane 1), BSA + nornicotine and glucose (lane 2), HSA (lane 3), HSA + nornicotine and glucose (lane 4). (b) Chemoluminescent development of Western blot from human plasma; HSA + nornicotine and glucose (lane 1), nonsmoker sample A total protein concentrations of 2.5 mg/ml (lane 2), 5.0 mg/ml (lane 3), 10 mg/ml (lane 4); nonsmoker sample B total protein concentrations of 2.5 mg/ml (lane 5), 5.0 mg/ml (lane 6), 10 mg/ml (lane 7); smoker sample C total protein concentrations of 2.5 mg/ml (lane 8), 5.0 mg/ml (lane 9), 10 mg/ml (lane 10); smoker sample D total protein concentrations of 2.5 mg/ml (lane 11), 5.0 mg/ml (lane 12), 10 mg/ml (lane 13). Reactions were performed by incubating sterile solutions (0.2-μm filter) of glucose (200 mM), nornicotine (0.8 mM), and protein (0.8 mM) in phosphate buffer (200 mM, pH 7.4) in the dark at 37°C.
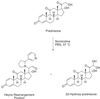
Fig 5.
Two products formed on reaction of nornicotine with prednisone, the prednisone Heyns rearrangement product, and 22-hydroxy-prednisone.
Similar articles
- Nornicotine aqueous aldol reactions: synthetic and theoretical investigations into the origins of catalysis.
Dickerson TJ, Lovell T, Meijler MM, Noodleman L, Janda KD. Dickerson TJ, et al. J Org Chem. 2004 Oct 1;69(20):6603-9. doi: 10.1021/jo048894j. J Org Chem. 2004. PMID: 15387581 - Receptor for advanced glycation end product (RAGE) upregulation in human gingival fibroblasts incubated with nornicotine.
Katz J, Caudle RM, Bhattacharyya I, Stewart CM, Cohen DM. Katz J, et al. J Periodontol. 2005 Jul;76(7):1171-4. doi: 10.1902/jop.2005.76.7.1171. J Periodontol. 2005. PMID: 16018761 - Hammett correlation of nornicotine analogues in the aqueous aldol reaction: implications for green organocatalysis.
Rogers CJ, Dickerson TJ, Brogan AP, Janda KD. Rogers CJ, et al. J Org Chem. 2005 Apr 29;70(9):3705-8. doi: 10.1021/jo050161r. J Org Chem. 2005. PMID: 15845010 - Glycation, carbonyl stress, EAGLEs, and the vascular complications of diabetes.
Lyons TJ. Lyons TJ. Semin Vasc Med. 2002 May;2(2):175-89. doi: 10.1055/s-2002-32041. Semin Vasc Med. 2002. PMID: 16222609 Review. - Smoking-induced metabolic disorders: a review.
Berlin I. Berlin I. Diabetes Metab. 2008 Sep;34(4 Pt 1):307-14. doi: 10.1016/j.diabet.2008.01.008. Epub 2008 May 12. Diabetes Metab. 2008. PMID: 18468932 Review.
Cited by
- Clinical and technical factors associated with skin intrinsic fluorescence in subjects with type 1 diabetes from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study.
Cleary PA, Braffett BH, Orchard T, Lyons TJ, Maynard J, Cowie C, Gubitosi-Klug RA, Way J, Anderson K, Barnie A, Villavicencio S; DCCT/EDIC Research Group. Cleary PA, et al. Diabetes Technol Ther. 2013 Jun;15(6):466-74. doi: 10.1089/dia.2012.0316. Diabetes Technol Ther. 2013. PMID: 23882708 Free PMC article. Clinical Trial. - A Role for Advanced Glycation End Products in Molecular Ageing.
Zgutka K, Tkacz M, Tomasiak P, Tarnowski M. Zgutka K, et al. Int J Mol Sci. 2023 Jun 8;24(12):9881. doi: 10.3390/ijms24129881. Int J Mol Sci. 2023. PMID: 37373042 Free PMC article. Review. - Nicotine alkaloid levels, and nicotine to nornicotine conversion, in Australian Nicotiana species used as chewing tobacco.
Moghbel N, Ryu B, Ratsch A, Steadman KJ. Moghbel N, et al. Heliyon. 2017 Dec 1;3(11):e00469. doi: 10.1016/j.heliyon.2017.e00469. eCollection 2017 Nov. Heliyon. 2017. PMID: 29264422 Free PMC article. - Tobacco cessation in primary care: maximizing intervention strategies.
Anczak JD, Nogler RA 2nd. Anczak JD, et al. Clin Med Res. 2003 Jul;1(3):201-16. doi: 10.3121/cmr.1.3.201. Clin Med Res. 2003. PMID: 15931310 Free PMC article. Review. - Glycation of the amyloid beta-protein by a nicotine metabolite: a fortuitous chemical dynamic between smoking and Alzheimer's disease.
Dickerson TJ, Janda KD. Dickerson TJ, et al. Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8182-7. doi: 10.1073/pnas.1332847100. Epub 2003 Jun 18. Proc Natl Acad Sci U S A. 2003. PMID: 12815102 Free PMC article.
References
- Maillard L. C. (1912) C. R. Acad. Sci. Ser. II 154, 66-68.
- Yaylayan V. A. & Huyghues-Despointes, A. (1994) Crit. Rev. Food Sci. Nutr. 34, 321-369. - PubMed
- Ledl F. & Schleicher, E. (1990) Angew. Chem. Int. Ed. Engl. 29, 565-594.
- Bucala R. & Cerami, A. (1996) in The Maillard Reaction: Consequences for the Chemical and Life Sciences, ed. Ikan, R. (Wiley, New York), pp. 161–181.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous