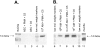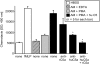Generation of C5a by phagocytic cells - PubMed (original) (raw)
Generation of C5a by phagocytic cells
Markus Huber-Lang et al. Am J Pathol. 2002 Nov.
Abstract
The complement activation product, C5a, is a powerful phlogistic factor. Using antibodies to detect human or rat C5a, incubation at pH 7.4 of human blood neutrophils or rat alveolar macrophages (AMs) with C5 in the presence of phorbol 12-myristate 13-acetate (PMA) led to generation of C5a. Rat AMs activated with lipopolysaccharide also generated C5a from C5. With activated neutrophils, extensive cleavage of C5 occurred, whereas activated macrophages had much more selective proteolytic activity for C5. Peripheral blood human or rat mononuclear cells and rat alveolar epithelial cells when stimulated with phorbol ester all failed to demonstrate an ability to cleave C5, suggesting a specificity of C5 cleavage by phagocytic cells. With rat AMs, C5a generation was time-dependent and was blocked if AMs were pretreated with inhibitors of transcription or protein synthesis (actinomycin D or cycloheximide). Similar treatment of activated human polymorphonuclear leukocytes only partially reduced C5a generation after addition of C5. C5a generated by activated AMs was biologically (chemotactically) active. This generation was sensitive to serine protease inhibitors but not to other classes of inhibitors. These data indicate that phagocytic cells, especially lung macrophages, can generate C5a from C5. In the context of the lung, this may represent an important C5a-generating pathway that is independent of the plasma complement system.
Figures
Figure 1.
Generation of C5a by activated human PMNs, human PBMCs, or rat AMs. Cells (1 × 107) were incubated at pH 7.4 with 90 μg of human C5 and (where indicated) PMA (100 ng/ml) or LPS (0.5 μg/ml) for 4 hours at 37°C and then Western blot analysis with anti-human C5a was performed. Positions of the molecular weight markers are shown at the extreme left region of the figure. Data are representative of three or more separate and independent experiments.
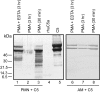
Figure 2.
Inability of PMA-stimulated rat AECs to generate C5a after incubation with 90 μg of C5 for 4 hours at 37°C. Conditions for Western blot analysis using anti-human C5a was similar to that described for Figure 1 ▶ . Data are representative of three or more separate and independent experiments.
Figure 3.
Ability of activated human PMNs or rat AMs to hydrolyze C5 using conditions similar to those described in Figure 1 ▶ except that anti-human C5 Ab was used for immunoblotting. Data are representative of two separate and independent experiments.
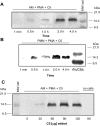
Figure 4.
A and B: Western blot detection of human C5a in supernatant fluids obtained up to 4 hours after addition of 90 μg of human C5 to 10 × 106 AMs (A) or 10 × 106 PMNs (B) in the presence of PMA (100 ng/ml). Positions of molecular weight markers are shown in the extreme right lane. C: Western blot analysis of supernatant fluids from PMA-stimulated rat AMs in the presence of increasing amounts (30 to 120 μg) of C5. Western blots in A, B, and C were all probed with anti-human C5a. Data are representative of four or more separate and independent experiments.
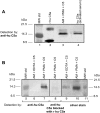
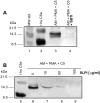
Figure 5.
A: Western blot analysis of various samples using anti-human C5a. Molecular weight markers are in lane 1 and recombinant human C5a in lane 2. The C5 cleavage product in supernatant fluids from the combination of PMAs, rat AMs, and C5 is shown in lane 3. C5a from activated human serum (lane 4) was first immunoprecipitated with anti-human C5a and then analyzed by Western blot analysis. B: Western blot analysis of AM-generated C5a. Molecular weight standards (lane 5); supernatant fluids from nonstimulated (10 mmol/L EDTA) rat AMs to which C5 was added (lane 6), and supernatant fluids from similarly treated rat AMs in the absence of EDTA (lane 7). Middle frame (lane 8): Antibody to human C5a was used as the immunodetecting Ab but preabsorbed with recombinant human C5a (10 μg/ml) and then used to detect the same products as shown in lane 7. Right frame: Silver stain of gel contained in lane 9 material similar to lane 6, whereas lane 10 contained material similar to that in lane 7. Reference molecular weight standards are in lane 11. Data are representative of two separate and independent experiments.
Figure 6.
Chemotactic activity for human PMNs using samples indicated in the inset. For these studies, 107 rat AMs were incubated with 90 μg of human C5a for 4 hours at 37°C. See text for details. The concentration of fMLP was 100 nmol/L. Statistical comparisons were to the supernatant fluids from PMA-stimulated AMs in the presence of C5 (black bar labeled “none”). IgG antibodies (10 μg/ml) to rat C5a or human C5a were added to supernatant fluids before chemotactic assays were performed. Data are representative of three separate and independent experiments.
Figure 7.
A: Western blot analysis using anti-human C5a Ab: lane 1, molecular weight standards; lane 2, recombinant human C5a (100 ng); lane 3, supernatant fluids from PMA-stimulated rat AMs to which C5 (90 μg) was added and incubation was performed at 37°C for 4 hours; lane 4, similar to lane 3 with the exception that at the start of incubation SBTI (100 μg/ml) was added. B: Western blot using the same anti-human C5a Ab: lane 5, human C5a; lanes 6 to 9, supernatant fluids from PMA-stimulated AMs to which C5 (90 μg) had been added in the presence of 0 to 100 μg of SLPI/ml. Data are representative of two separate and independent experiments.
Figure 8.
Ability of activated AMs and PMNs to generate C5a in the presence of actinomycin D and cycloheximide using conditions similar to those described in Figure 1 ▶ . Details are provided in the text. Western blots were probed with anti-human C5a Ab. Data are representative of two separate and independent experiments.
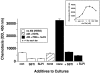
Figure 9.
Chemotactic activity for human PMNs in samples indicated in the top left inset. Most details are similar to those described in the legend to Figure 6 ▶ . In some cases, SBTI (100 μg/ml) or SLPI (100 μg/ml) were added to the rat AM suspensions before addition of PMA and C5. A dose response for neutrophil chemotactic responses to recombinant human C5a (0.01 to 1000 nmol/L) is shown in the top right inset. Data are representative of three separate and independent experiments.
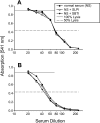
Figure 10.
Hemolytic activity (CH50) of rat (A) or human (B) serum in the absence or presence of fixed concentrations of SBTI (100 μg/ml) or SLPI (100 μg/ml) added to various dilutions of serum. Dotted lines indicate the 50% hemolysis values. Data are representative of two separate and independent experiments.
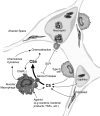
Figure 11.
Proposed model of locally generated C5a in the alveolar compartment. C5 derived from the vascular compartment or produced locally from various types of lung cells is cleaved by activated lung macrophages to generate biologically active C5a. The cleavage enzyme from macrophages appears to be an inducible serine protease that can be blocked by naturally occurring SLPI. After C5 cleavage, C5a causes neutrophils to migrate into the alveolar space, resulting in release of inflammatory mediators, oxidative products, and other damaging proteases. C5a may also stimulate in an autocrine manner lung macrophages to enhance the inflammatory response.
Similar articles
- Complement activation in cystic fibrosis respiratory fluids: in vivo and in vitro generation of C5a and chemotactic activity.
Fick RB Jr, Robbins RA, Squier SU, Schoderbek WE, Russ WD. Fick RB Jr, et al. Pediatr Res. 1986 Dec;20(12):1258-68. doi: 10.1203/00006450-198612000-00014. Pediatr Res. 1986. PMID: 3540828 - Cleavage of the fifth component of human complement and release of a split product with C5a-like activity by crystalline silica through free radical generation and kallikrein activation.
Governa M, Fenoglio I, Amati M, Valentino M, Bolognini L, Coloccini S, Volpe AR, Carmignani M, Fubini B. Governa M, et al. Toxicol Appl Pharmacol. 2002 Mar 15;179(3):129-36. doi: 10.1006/taap.2002.9351. Toxicol Appl Pharmacol. 2002. PMID: 11906242 - Pulmonary alveolar type II epithelial cells synthesize and secrete proteins of the classical and alternative complement pathways.
Strunk RC, Eidlen DM, Mason RJ. Strunk RC, et al. J Clin Invest. 1988 May;81(5):1419-26. doi: 10.1172/JCI113472. J Clin Invest. 1988. PMID: 2966814 Free PMC article. - Cathepsin D is released after severe tissue trauma in vivo and is capable of generating C5a in vitro.
Huber-Lang M, Denk S, Fulda S, Erler E, Kalbitz M, Weckbach S, Schneider EM, Weiss M, Kanse SM, Perl M. Huber-Lang M, et al. Mol Immunol. 2012 Feb;50(1-2):60-5. doi: 10.1016/j.molimm.2011.12.005. Epub 2012 Jan 14. Mol Immunol. 2012. PMID: 22244896 - Biologically active complement (C5)-derived peptides and their relevance to disease.
Perez HD. Perez HD. Crit Rev Oncol Hematol. 1984;1(3):199-225. doi: 10.1016/s1040-8428(84)80012-8. Crit Rev Oncol Hematol. 1984. PMID: 6241542 Review.
Cited by
- C5aR1 Activation Drives Early IFN-γ Production to Control Experimental Toxoplasma gondii Infection.
Briukhovetska D, Ohm B, Mey FT, Aliberti J, Kleingarn M, Huber-Lang M, Karsten CM, Köhl J. Briukhovetska D, et al. Front Immunol. 2020 Jul 8;11:1397. doi: 10.3389/fimmu.2020.01397. eCollection 2020. Front Immunol. 2020. PMID: 32733463 Free PMC article. - Change in the immune function of porcine iliac artery endothelial cells infected with porcine circovirus type 2 and its inhibition on monocyte derived dendritic cells maturation.
Yang N, Qiao J, Liu S, Zou Z, Zhu L, Liu X, Zhou S, Li H. Yang N, et al. PLoS One. 2017 Oct 26;12(10):e0186775. doi: 10.1371/journal.pone.0186775. eCollection 2017. PLoS One. 2017. PMID: 29073194 Free PMC article. - Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a.
Ali H. Ali H. Immunol Lett. 2010 Jan 18;128(1):36-45. doi: 10.1016/j.imlet.2009.10.007. Epub 2009 Nov 4. Immunol Lett. 2010. PMID: 19895849 Free PMC article. Review. - Neutrophil homeostasis and inflammation: novel paradigms from studying periodontitis.
Hajishengallis G, Chavakis T, Hajishengallis E, Lambris JD. Hajishengallis G, et al. J Leukoc Biol. 2015 Oct;98(4):539-48. doi: 10.1189/jlb.3VMR1014-468R. Epub 2014 Dec 29. J Leukoc Biol. 2015. PMID: 25548253 Free PMC article. Review. - Complement C3 serum levels in anorexia nervosa: a potential biomarker for the severity of disease?
Flierl MA, Gaudiani JL, Sabel AL, Long CS, Stahel PF, Mehler PS. Flierl MA, et al. Ann Gen Psychiatry. 2011 May 4;10:16. doi: 10.1186/1744-859X-10-16. Ann Gen Psychiatry. 2011. PMID: 21542928 Free PMC article.
References
- Ishii Y, Kobayashi J, Kitamura S: Chemotactic factor generation and cell accumulation in acute lung injury induced by endotracheal acid instillation. Prostaglandins Leukot Essent Fatty Acids 1989, 37:65-70 - PubMed
- Sibille Y, Reynolds HY: Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Med 1990, 141:471-501 - PubMed
- Solomkin JS, Cotta LA, Satoh PS, Hurst JM, Nelson RD: Complement activation and clearance in acute illness and injury: evidence for C5a as a cell-directed mediator of the adult respiratory distress syndrome in man. Surgery 1984, 97:668-678 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL031963/HL/NHLBI NIH HHS/United States
- HL-31963/HL/NHLBI NIH HHS/United States
- R37 GM029507/GM/NIGMS NIH HHS/United States
- GM-29507/GM/NIGMS NIH HHS/United States
- R01 GM029507/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous