Altered kinetics and benzodiazepine sensitivity of a GABAA receptor subunit mutation [gamma 2(R43Q)] found in human epilepsy - PubMed (original) (raw)
. 2002 Nov 12;99(23):15170-5.
doi: 10.1073/pnas.212320199. Epub 2002 Nov 1.
David A Wagner, Cynthia Czajkowski, Brett A Cromer, Michael W Parker, Robyn H Wallace, Louise A Harkin, John C Mulley, Carla Marini, Samuel F Berkovic, David A Williams, Mathew V Jones, Steven Petrou
Affiliations
- PMID: 12415111
- PMCID: PMC137562
- DOI: 10.1073/pnas.212320199
Altered kinetics and benzodiazepine sensitivity of a GABAA receptor subunit mutation [gamma 2(R43Q)] found in human epilepsy
David N Bowser et al. Proc Natl Acad Sci U S A. 2002.
Abstract
The gamma-aminobutyric acid type A (GABA(A)) receptor mediates fast inhibitory synaptic transmission in the CNS. Dysfunction of the GABA(A) receptor would be expected to cause neuronal hyperexcitability, a phenomenon linked with epileptogenesis. We have investigated the functional consequences of an arginine-to-glutamine mutation at position 43 within the GABA(A) gamma(2)-subunit found in a family with childhood absence epilepsy and febrile seizures. Rapid-application experiments performed on receptors expressed in HEK-293 cells demonstrated that the mutation slows GABA(A) receptor deactivation and increases the rate of desensitization, resulting in an accumulation of desensitized receptors during repeated, short applications. In Xenopus laevis oocytes, two-electrode voltage-clamp analysis of steady-state currents obtained from alpha(1)beta(2)gamma(2) or alpha(1)beta(2)gamma(2)(R43Q) receptors did not reveal any differences in GABA sensitivity. However, differences in the benzodiazepine pharmacology of mutant receptors were apparent. Mutant receptors expressed in oocytes displayed reduced sensitivity to diazepam and flunitrazepam but not the imidazopyridine zolpidem. These results provide evidence of impaired GABA(A) receptor function that could decrease the efficacy of transmission at inhibitory synapses, possibly generating a hyperexcitable neuronal state in thalamocortical networks of epileptic patients possessing the mutant subunit.
Figures
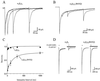
Fig 1.
The γ2(R43Q) mutation slows deactivation and enhances fast desensitization. (A) Normalized average currents evoked by applying 2-ms pulses of saturating GABA (10 mM) to outside-out patches. Actual peak currents were 1.1 nA (WT) and 1.3 nA (R43Q). (B) Normalized average currents evoked by 500-ms pulses of saturating GABA. Actual peak currents were 230 pA (WT) and 1.2 nA (R43Q). (C and D) Comparison of kinetics in the mutant with those in receptors lacking the γ2-subunit. Traces from the mutant are the same as those in A and B. The actual peak currents for α1β2 were 305 pA (C) and 270 pA (D). The top traces in each graph are the liquid junction currents recorded at the open pipette tip immediately after each experiment to evaluate the speed of solution exchange (20–80% exchange times were <200 μs). Deactivation time constants (and percent amplitude) were (in mean ± SEM) 9 ± 1 ms (74 ± 3%) and 148 ± 42 ms for α1β2γ2; 8 ± 0.9 ms (52 ± 3.1%), 64 ± 8 ms (30 ± 2%), and 415 ± 49 ms for α1β2γ2(R43Q); 14 ± 0.9 ms (70 ± 3%), 83 ± 6 ms (21 ± 2%), and 664 ± 103 ms for α1β2.
Fig 2.
The γ2(R43Q) mutation increases paired-pulse desensitization. A (WT) and B (R43Q) each show three overlaid responses to pairs of 2-ms pulses of 10 mM GABA separated by 30, 100, or 300 ms. Each trace is the average of five records. (C) Results of paired-pulse experiments plotted as fractional recovery vs. interpulse interval. Recovery is calculated as: (I2 − B2)/(I1 − B2), where I1 and I2 are the peak responses to the first and second GABA pulses and B2 is the current immediately before the second GABA pulse. The data for R43Q receptors were fit with a biexponential curve that had time constants of 0.16 s (57%) and 4.2 s (31%) and a y intercept of 0.12. The data for WT receptors were fit with a monoexponential curve that had a time constant of 0.13 s (9%) and a y intercept of 0.91. (D) Responses of α1β2 and α1β2γ2(R43Q) receptors to 2-ms pulses of 10 mM GABA in the presence or absence of 10 μM Zn2+ applied 500 ms before GABA application. Zn2+ (10 μM) blocks currents from α1β2 receptors strongly but blocks currents from α1β2γ2(R43Q) receptors weakly, indicating efficient incorporation of the γ2(R43Q) subunit when cotransfected with α1- and β2-subunit cDNAs.
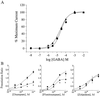
Fig 3.
(A) Responsiveness to GABA is not altered in oocytes expressing the γ2(R43Q) subunit. GABA dose-response curves for oocytes expressing WT (•) and R43Q (▴) γ2-subunits with WT α1- and β2-subunits in a 1:1:10 molar ratio. Data points represent the mean current ± SE from five or more oocytes from two or more frogs. Data were fitted to a curve following the equation % max = min + (Max − Min)/(1+10[(logEC50−X)⋅_n_]), where Max is the maximal current, Min is the current at 0.01 μM GABA, X is the log of the GABA concentration, EC50 is the half-maximal current response, and n is the Hill coefficient. The vertical, dashed lines show the GABA concentrations used in our earlier (1 μM) and present (10 μM) studies of benzodiazepine potentiation. (B) Oocytes expressing γ2(R43Q) have reduced sensitivity to potentiation by the classical benzodiazepines diazepam and flunitrazepam but not zolpidem. Dose-response curves were constructed for oocytes expressing WT (•) and R43Q (▴) γ2-subunits in conjunction with WT α1- and β2-subunits in a 1:1:10 molar ratio. Currents were elicited with 10 μM GABA. Each data point is the median ratio of the current in the presence of GABA alone and GABA plus BDZ of at least four oocytes from two or more frogs (error bars indicate SEM). Predicted curves are drawn through the points based on the median fitted parameters; the asterisk marks where the predicted curves diverge with 95% confidence. The median EC50 values were 140 nM (WT) and 44 nM (R43Q) for diazepam, 24 nM (WT) and 16 nM (R43Q) for flunitrazepam, and 15 nM (WT) and 30 nM (R43Q) for zolpidem. Hill slopes were not significantly different from unity.
Similar articles
- Inhibition of GABAA receptor function by tyrosine kinase inhibitors and their inactive analogues.
Dunne EL, Moss SJ, Smart TG. Dunne EL, et al. Mol Cell Neurosci. 1998 Nov;12(4-5):300-10. doi: 10.1006/mcne.1998.0717. Mol Cell Neurosci. 1998. PMID: 9828093 - Molecular analysis of the A322D mutation in the GABA receptor alpha-subunit causing juvenile myoclonic epilepsy.
Krampfl K, Maljevic S, Cossette P, Ziegler E, Rouleau GA, Lerche H, Bufler J. Krampfl K, et al. Eur J Neurosci. 2005 Jul;22(1):10-20. doi: 10.1111/j.1460-9568.2005.04168.x. Eur J Neurosci. 2005. PMID: 16029191 - Properties of recombinant gamma-aminobutyric acid A receptor isoforms containing the alpha 5 subunit subtype.
Burgard EC, Tietz EI, Neelands TR, Macdonald RL. Burgard EC, et al. Mol Pharmacol. 1996 Jul;50(1):119-27. Mol Pharmacol. 1996. PMID: 8700104 - [Implications of GABAergic synapses in neuropsychiatry].
Lloyd KG, Perrault G, Zivkovic B. Lloyd KG, et al. J Pharmacol. 1985;16 Suppl 2:5-27. J Pharmacol. 1985. PMID: 2867252 Review. French. - [Intervention of GABAergic neurotransmission in partial epilepsies].
Bureau M, Laschet J, Minier F, Chauvel P. Bureau M, et al. Rev Neurol (Paris). 1997;153 Suppl 1:S46-54. Rev Neurol (Paris). 1997. PMID: 9686248 Review. French.
Cited by
- Mutation Screening of the γ-Aminobutyric Acid Type-A Receptor Subunit γ2 Gene in Korean Patients with Childhood Absence Epilepsy.
Kim YO, Kim MK, Nam TS, Jang SY, Park KW, Kim EY, Rho YI, Woo YJ. Kim YO, et al. J Clin Neurol. 2012 Dec;8(4):271-5. doi: 10.3988/jcn.2012.8.4.271. Epub 2012 Dec 21. J Clin Neurol. 2012. PMID: 23323135 Free PMC article. - Functional characterization of the 1,5-benzodiazepine clobazam and its major active metabolite N-desmethylclobazam at human GABA(A) receptors expressed in Xenopus laevis oocytes.
Hammer H, Ebert B, Jensen HS, Jensen AA. Hammer H, et al. PLoS One. 2015 Mar 23;10(3):e0120239. doi: 10.1371/journal.pone.0120239. eCollection 2015. PLoS One. 2015. PMID: 25798598 Free PMC article. - GABAA Receptor β2E155 Residue Located at the Agonist-Binding Site Is Involved in the Receptor Gating.
Jatczak-Śliwa M, Kisiel M, Czyzewska MM, Brodzki M, Mozrzymas JW. Jatczak-Śliwa M, et al. Front Cell Neurosci. 2020 Feb 11;14:2. doi: 10.3389/fncel.2020.00002. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32116555 Free PMC article. - Design, synthesis, and subtype selectivity of 3,6-disubstituted β-carbolines at Bz/GABA(A)ergic receptors. SAR and studies directed toward agents for treatment of alcohol abuse.
Yin W, Majumder S, Clayton T, Petrou S, VanLinn ML, Namjoshi OA, Ma C, Cromer BA, Roth BL, Platt DM, Cook JM. Yin W, et al. Bioorg Med Chem. 2010 Nov 1;18(21):7548-64. doi: 10.1016/j.bmc.2010.08.049. Epub 2010 Sep 29. Bioorg Med Chem. 2010. PMID: 20888240 Free PMC article. - A Systems Biology Approach for Personalized Medicine in Refractory Epilepsy.
Naimo GD, Guarnaccia M, Sprovieri T, Ungaro C, Conforti FL, Andò S, Cavallaro S. Naimo GD, et al. Int J Mol Sci. 2019 Jul 30;20(15):3717. doi: 10.3390/ijms20153717. Int J Mol Sci. 2019. PMID: 31366017 Free PMC article. Review.
References
- Berkovic S. F. & Scheffer, I. E. (2001) Epilepsia 42, 16-23. - PubMed
- Lerche H., Jurkat-Rott, K. & Lehmann-Horn, F. (2001) Am. J. Med. Genet. 106, 146-159. - PubMed
- Wallace R. H., Wang, D. W., Singh, R., Scheffer, I. E., George, A. L., Jr., Phillips, H. A., Saar, K., Reis, A., Johnson, E. W., Sutherland, G. R., et al. (1998) Nat. Genet. 19, 366-370. - PubMed
- Charlier C., Singh, N. A., Ryan, S. G., Lewis, T. B., Reus, B. E., Leach, R. J. & Leppert, M. (1998) Nat. Genet. 18, 53-55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous