The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function - PubMed (original) (raw)
The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function
Charlotte Helfrich-Förster et al. J Neurosci. 2002.
Abstract
Circadian rhythms can be entrained by light to follow the daily solar cycle. In Drosophila melanogaster a pair of extraretinal eyelets expressing immunoreactivity to Rhodopsin 6 each contains four photoreceptors located beneath the posterior margin of the compound eye. Their axons project to the region of the pacemaker center in the brain with a trajectory resembling that of Bolwig's organ, the visual organ of the larva. A lacZ reporter line driven by an upstream fragment of the developmental gap gene Krüppel is a specific enhancer element for Bolwig's organ. Expression of immunoreactivity to the product of lacZ in Bolwig's organ persists through pupal metamorphosis and survives in the adult eyelet. We thus demonstrate that eyelet derives from the 12 photoreceptors of Bolwig's organ, which entrain circadian rhythmicity in the larva. Double labeling with anti-pigment-dispersing hormone shows that the terminals of Bolwig's nerve differentiate during metamorphosis in close temporal and spatial relationship to the ventral lateral neurons (LN(v)), which are essential to express circadian rhythmicity in the adult. Bolwig's organ also expresses immunoreactivity to Rhodopsin 6, which thus continues in eyelet. We compared action spectra of entrainment in different fly strains: in flies lacking compound eyes but retaining eyelet (so(1)), lacking both compound eyes and eyelet (so(1);gl(60j)), and retaining eyelet but lacking compound eyes as well as cryptochrome (so(1);cry(b)). Responses to phase shifts suggest that, in the absence of compound eyes, eyelet together with cryptochrome mainly mediates phase delays. Thus a functional role in circadian entrainment first found in Bolwig's organ in the larva is retained in eyelet, the adult remnant of Bolwig's organ, even in the face of metamorphic restructuring.
Figures
Fig. 1.
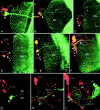
Bolwig's nerve and the eyelet tract both have a close spatial relationship to the lateral neurons in the developing optic lobe. Shown are confocal images of the right optic lobe in frontal view. Green channel, Photoreceptors (gmr_-driven expression of GFP in_A–F; antibody 24B10 in G–I);red channel, lateral neurons (anti-PDH). Some ommatidia of the developing retina are visible in D and_E_; other images depict the terminals of photoreceptors R1–R6 in the lamina (La) and R7, R8 in the second neuropil, the medulla (Me). In larvae (A) and P + 20% pupae (B), Bolwig's nerve (BN) traverses the medulla and terminates directly in the larval optic neuropil (arrowhead), which transforms into the accessory medulla during metamorphosis. In the larval optic neuropil the terminals overlap putative dendrites from the small and large lateral neurons (s-LNv and l-LNv). Additional fibers parallel to Bolwig's nerve (small green arrows) are possibly neurites of tangential neurons. Expression in the latter persists in early pupae (B) when expression in Bolwig's nerve starts to disappear (C). At approximately P + 50% (D) faint labeling first appears in the eyelet tract (Ey-Tr) together with the first labeling in l-LNv. At P + 80% (E) the labeling pattern mainly resembles that of adult flies (F). The tract from eyelet now shows a ventral extension overlapping arborizations of the l-LNv(arrowheads). Fibers arising from l-LNv form a network over the surface of the medulla in close vicinity to the tract from eyelet (arrow in F). Bolwig's tract (G) and eyelet (H) are present in eyeless sine oculis (so 1) mutants contacting the LNv. _so1_flies with tiny eye remnants possess, in addition, a well established axonal connection of their ommatidial photoreceptors to the medulla (I). Scale bar: (in I)A–I, 20 μm.
Fig. 2.
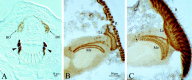
Bolwig's organ and the developing projection of eyelet. A, Frontal 8 μm wax section of anterior region of third instar larva showing immunoreactivity to Rhodopsin 6 in Bolwig's organ (BO), situated dorsally to the cephalopharyngeal skeleton (arrowheads). Differential interference contrast microscopy was used.B, C, Horizontal cryostat sections 10 μm thick of pupal optic lobe immunolabeled with antibody 24B10.B, Pupa at approximately P + 45%. In early pupal stages the developing medulla (Me) lies orthogonal to the lamina (La), and the photoreceptor terminals of eyelet are not seen. C, Pupa at approximately P + 70%. At approximately P + 50% the medulla rotates, becoming concentric with the lamina, and the terminals of eyelet immunolabel at a position anterior to the medulla neuropil (arrow) corresponding to the accessory medulla. R, Retina. Cell bodies are not easily seen in this preparation. In the late pupa and adult the axons from the cells of eyelet project through the outer chiasm, and their terminals in the accessory medulla all label with photoreceptor-specific antibodies. Scale bars: A, 10 μm; (in B) B, C, 50 μm.
Fig. 3.
Krüppel_-driven_lacZ expression in Bolwig's organ and nerve persists throughout metamorphosis. Bolwig's organ and nerve throughout pupal metamorphosis are detected by immunoreactivity to β-gal in the transgenic line BS23 (Kr-BO-lacZ) in which_lacZ_ expression is driven by the promoter for_Krüppel_. A, Dorsal view of both eye discs and hemispheres in a whole-mount preparation revealing Bolwig's organ (arrowhead) and the trajectory of its nerve (BN) in the white prepupa. B–D, Horizontal Vibratome slices (100 μm) of the right hemisphere of the pupal optic lobe at different stages: B, P + 20%;C, P + 40%; D, P + 80%. Persistent β-gal expression reveals that Bolwig's organ is transformed into eyelet, the axon bundle of which is shown relative to the lamina (La) and medulla (Me) neuropils (in_D_), retina (R in B,D), and basement membrane (arrows in_D_). Cell number in Bolwig's organ decreases from 12 (inset, A) to four in the adult (inset, D) after separation from the mouthhooks at P + 1 hr (inset, B) and retraction of the cell bodies that are distributed in a trail of two clusters by P + 5% (inset, C). Scale bars: A, 250 μm; B, C,inset in C, 50 μm; D,insets in A and B, 25 μm; inset in D, 20 μm.
Fig. 4.
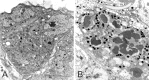
Ultrastructural differentiation of eyelet. Shown are the photoreceptors of eyelet at different stages of differentiation in pupal (A) and adult (B) flies. A, Pupa with amber eyes corresponding to approximately P + 60% (Bainbridges and Bownes, 1981). The first microvilli of developing rhabdomeres have become distinct, but no pigment is yet visible. B, Fully differentiated eyelet photoreceptors with rhabdomeres and black pigment granules.Arrows show developing microvillar structures in_A_ and fully developed rhabdomeres in B. Scale bar, 1 μm.
Fig. 5.
Entrainment of flies by spectral light. Shown are representative actograms from wild-type flies (WT), so 1 flies, and_so1;gl60j_flies subjected to 12 hr light/dark cycles (LD) of different wavelengths, the phase of which was advanced twice by 6 hr. One-half of the flies subsequently were recorded under constant darkness (DD). Light was blue-green (top left panel, 460–540 nm), red (top right panel, >600 nm), blue (middle and bottom left panels, 420 nm), or green (middle and bottom right panels, 486 nm). Irradiances are indicated in the_margin_ in photons · cm−2 · sec−1. Wild-type flies re-entrained quickly to the 6 hr phase advances, showing bimodal activity patterns with morning and evening peaks. Activity was restricted mainly to the light phase of the LD cycle. Mutants extended activity into the dark phase and needed several days to re-entrain to phase advances. Some flies failed to re-entrain to green light (_so1;gl60j_and so1 after second phase advance). The morning peak was visible in so1_flies only during the blue LD cycle after the first phase shift and faintly during the green LD cycle before the first phase shift. The_so1 fly in green light showed antidromic phase shifting.
Fig. 6.
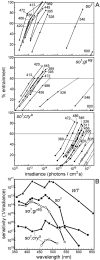
Dose–response curves for re-entrainment and corresponding action spectra. The dose–response curves (A) for so 1,so1;gl60j, and_so1;cryb_flies show the percentage of re-entrained flies for all wavelengths as a function of the irradiance. For_so1;cryb_mutants both the flies with eye remnants (●) and completely eyeless flies (○) were tested. From left to_right_, the wavelengths for eyeless flies were 472, 486, 517, and 456 nm. Action spectra (B) were derived from these dose–response curves by determining the light intensities that were necessary to entrain 60% of the flies at each wavelength. The action spectrum for wild-type flies is derived from data byBlaschke et al. (1996).
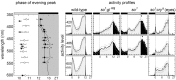
Fig. 7.
Phase plot of evening activity peaks and average activity profiles of entrained flies at different wavelengths. The_black_ and white bars along the top of each plot indicate the light/dark regimen. The maxima of the evening peaks occurred before lights-off in wild-type flies (▴) and in_so_ 1 ;cryb_mutants with eye remnants (○) but after lights-off in both_so1;gl60j(●) and so1 (■) mutants. Average activity profiles at wavelengths of 420, 486, and 600 nm reveal that the activity of wild-type flies and_so1;cryb_mutants with eye remnants is restricted mainly to the light phase of the LD cycle, whereas it extends into the dark phase in the eyeless mutants so1 and_so1;gl60j_(black portion of the curves). In_so1_ mutants the phase of the evening peak was dependent on the wavelength and occurred later at longer wavelengths (see phase plot and arrows in the activity profiles of so1 flies). Except for a single fly, the eyeless so1 and_so1;gl60j_were not entrainable at 600 nm, so average activity profiles could not be calculated for these flies at that wavelength. ZT, Zeitgeber time (ZT 0 = lights-on; ZT 12 = lights-off).
Similar articles
- Photic input pathways that mediate the Drosophila larval response to light and circadian rhythmicity are developmentally related but functionally distinct.
Hassan J, Iyengar B, Scantlebury N, Rodriguez Moncalvo V, Campos AR. Hassan J, et al. J Comp Neurol. 2005 Jan 17;481(3):266-75. doi: 10.1002/cne.20383. J Comp Neurol. 2005. PMID: 15593374 - The control of cell fate in the embryonic visual system by atonal, tailless and EGFR signaling.
Daniel A, Dumstrei K, Lengyel JA, Hartenstein V. Daniel A, et al. Development. 1999 Jul;126(13):2945-54. doi: 10.1242/dev.126.13.2945. Development. 1999. PMID: 10357938 - Light input pathways to the circadian clock of insects with an emphasis on the fruit fly Drosophila melanogaster.
Helfrich-Förster C. Helfrich-Förster C. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020 Mar;206(2):259-272. doi: 10.1007/s00359-019-01379-5. Epub 2019 Nov 5. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020. PMID: 31691095 Free PMC article. Review. - Nonvisual photoreceptors of the deep brain, pineal organs and retina.
Vigh B, Manzano MJ, Zádori A, Frank CL, Lukáts A, Röhlich P, Szél A, Dávid C. Vigh B, et al. Histol Histopathol. 2002 Apr;17(2):555-90. doi: 10.14670/HH-17.555. Histol Histopathol. 2002. PMID: 11962759 Review.
Cited by
- Communication Among Photoreceptors and the Central Clock Affects Sleep Profile.
Damulewicz M, Ispizua JI, Ceriani MF, Pyza EM. Damulewicz M, et al. Front Physiol. 2020 Aug 11;11:993. doi: 10.3389/fphys.2020.00993. eCollection 2020. Front Physiol. 2020. PMID: 32848895 Free PMC article. - Drosophila TRP channels and animal behavior.
Fowler MA, Montell C. Fowler MA, et al. Life Sci. 2013 Mar 19;92(8-9):394-403. doi: 10.1016/j.lfs.2012.07.029. Epub 2012 Aug 1. Life Sci. 2013. PMID: 22877650 Free PMC article. Review. - The HisCl1 histamine receptor acts in photoreceptors to synchronize Drosophila behavioral rhythms with light-dark cycles.
Alejevski F, Saint-Charles A, Michard-Vanhée C, Martin B, Galant S, Vasiliauskas D, Rouyer F. Alejevski F, et al. Nat Commun. 2019 Jan 16;10(1):252. doi: 10.1038/s41467-018-08116-7. Nat Commun. 2019. PMID: 30651542 Free PMC article. - Gravisensation and modulation of gravitactic responses by other sensory cues in the monarch butterfly (Danaus plexippus).
Kendzel MJ, Parlin AF, Guerra PA. Kendzel MJ, et al. J Exp Biol. 2023 Nov 1;226(21):jeb245451. doi: 10.1242/jeb.245451. Epub 2023 Nov 7. J Exp Biol. 2023. PMID: 37818736 Free PMC article. - Multilevel regulation of the glass locus during Drosophila eye development.
Fritsch C, Bernardo-Garcia FJ, Humberg TH, Mishra AK, Miellet S, Almeida S, Frochaux MV, Deplancke B, Huber A, Sprecher SG. Fritsch C, et al. PLoS Genet. 2019 Jul 12;15(7):e1008269. doi: 10.1371/journal.pgen.1008269. eCollection 2019 Jul. PLoS Genet. 2019. PMID: 31299050 Free PMC article.
References
- Adler K. Extraocular photoreception in amphibians. Photochem Photobiol. 1976;23:275–298. - PubMed
- Bainbridges SP, Bownes M. Staging the metamorphosis of Drosophila melanogaster. J Embryol Exp Morphol. 1981;66:57–80. - PubMed
- Bennett MF. Extraocular light receptors and circadian rhythms. In: Autrum H, editor. Handbook of sensory physiology, Vol VII/6A. Springer; Berlin: 1979. pp. 641–663.
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. - PubMed
- Blanchardon E, Grima B, Klarsfeld A, Chélot E, Hardin PE, Préat T, Rouyer F. Defining the role of Drosophila lateral neurons in the control of circadian activity and eclosion rhythms by targeted genetic ablation and PERIOD protein overexpression. Eur J Neurosci. 2001;13:871–888. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous