Purification of polyglutamine aggregates and identification of elongation factor-1alpha and heat shock protein 84 as aggregate-interacting proteins - PubMed (original) (raw)
Purification of polyglutamine aggregates and identification of elongation factor-1alpha and heat shock protein 84 as aggregate-interacting proteins
Kenichi Mitsui et al. J Neurosci. 2002.
Abstract
Aggregates of green fluorescent protein (GFP)-fused truncated N-terminal huntingtin containing abnormally long polyglutamine tracts (150 repeats of glutamine residue) were purified from an ecdysone-inducible mutant neuro2A cell line (HD150Q-28) by using a fluorescence-activated cell sorter. To analyze the aggregate-interacting proteins, we subjected the purified aggregates to SDS-PAGE; prominent protein bands in the gel were digested with Achromobactor lysyl endopeptidase, followed by a HPLC-mass spectrometry (MS) analysis. The resulting data of tandem MS analysis revealed that, in addition to ubiquitin and widely reported chaperone proteins such as heat shock cognate 70 (HSC70), human DNA J-1 (HDJ-1), and HDJ-2, the translational elongation factor-1alpha (EF-1alpha) and heat shock protein 84 (HSP84) also were recognized as aggregate-interacting proteins. Sequestration of these proteins to aggregates was confirmed further by several immunochemical methods. We confirmed that, in addition to the other known proteins, EF-1alpha and HSP84 also colocalized with the intracellular aggregates. An assay of the transient expression of EF-1alpha and HSP84 in HD150Q-28 cells revealed that both proteins improved cell viability. Moreover, the rate of aggregate formation decreased in both transfectants. Our study suggests that both EF-1alpha and HSP84 are involved in the neurodegenerative process of polyglutamine diseases.
Figures
Fig. 1.
Expression of tNhtt-16Q-GFP and tNhtt-150Q-GFP in differentiated N2A cells. HD16Q-23 (a, b) and HD150Q-28 (c, d) were treated with db-cAMP and ponasterone A. After 2 d of culture their morphology and tNhtt-GFP expressions were observed in phase contrast (a, c) and corresponding fluorescence (b, d) images. Scale bars, 20 μm.
Fig. 2.
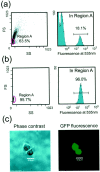
Purification of tNhtt-150Q-GFP aggregates with a cell sorter. a, Sorting profiles of aggregates from HD150Q-28 lysate are shown in a scattergram [left; forward scattering (FS) vs side scattering (SS)] and in a histogram for the fluorescence at 535 nm (right). Aggregates with bright fluorescence are observed mostly in the particles within Region A of the_left panel_. Numbers in the_left_ and right panels are the relative frequency of particles in Region A against the total particles and the frequency of bright particles in Region A, respectively. b, Results of the reanalysis of the sorted aggregates. Note that sorted particles are homogeneous with respect to the forward scattering, side scattering, and the fluorescence at 535 nm. c, Microscopic observation of purified aggregates presented by phase contrast image (left) and corresponding fluorescence image (right). Scale bars, 5 μm.
Fig. 3.
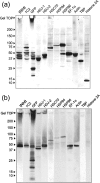
SDS-PAGE of purified aggregates and protein assignment to the CBB-stained bands. Approximately 17,000 purified aggregates were subjected to SDS-PAGE. Coomassie blue-stained bands in the gel were excised, followed by in-gel digestion with LysC. HPLC-MS/MS analysis of the enzyme-digested gel extracts assigned the indicated proteins to the corresponding bands. The white arrowheads with question marks indicate the protein bands that were applied to the HPLC-MS analysis but that could not be identified in this study.
Fig. 4.
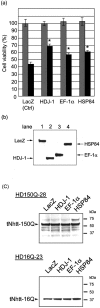
Western blot analysis of AIP candidates. HD150Q-28 cell lysate (a) and purified aggregates (b) were subjected to SDS-PAGE, followed by Western blotting probed with antibodies against the proteins that are indicated on the top of each panel. HRP-conjugated secondary antibodies were used, and the signal was detected by ECL.
Fig. 5.
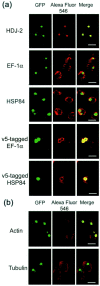
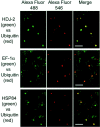
Immunocytochemical analysis of AIP candidates in HD150Q-28 cells. Cells of 2 d in culture after differentiation and tNhtt-150Q-GFP induction were used. Fixed cells were incubated with antibodies against the proteins that are indicated at the_left_ and then were labeled with Alexa Fluor 546-labeled anti-rabbit or anti-mouse secondary antibodies. Colocalization (a) and uncolocalization (b) of AIP candidates with aggregates are presented. Left column, Fluorescence of aggregates of tNhtt-150Q-GFP. Middle column, Alexa Fluor 546-labeled proteins. Right column, Merged images of the two signals. Scale bars, 20 μm.
Fig. 6.
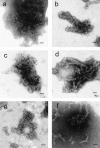
Immunogold labeling of AIP candidates on purified aggregates. The ultrastructure of purified aggregates was observed by electron microscope (a). For immunogold labeling the aggregates were treated with antibodies against HDJ-2 (b), EF-1α (c), HSP84 (d), actin (e), and tubulin (f), followed by probing with immunogold-conjugated anti-IgG antibodies. Black dots on the aggregate fibers represent the labeled gold particles. Scale bars, 30 nm.
Fig. 7.
Immunohistochemical analysis of aggregate-interacting candidates in an R6/2 transgenic mouse brain. Frozen brain sections of an R6/2 transgenic mouse were double labeled with anti-ubiquitin antibody, together with the antibodies against the indicated proteins at the left. Anti-ubiquitin antibody was probed with Alexa Fluor 546-labeled secondary antibody, and the antibodies against the indicated proteins were probed with Alexa Fluor 488-labeled secondary antibody. Left column, The Alexa Fluor 488-labeled proteins. Middle column, The localization of Alexa Fluor 546-labeled ubiquitin. Right column, The merged images. Scale bars, 20 μm.
Fig. 8.
Reduction of polyQ-mediated cellular toxicity in HSP84- and EF-1α-overexpressing mutant N2A cells. a, HD150Q-28 cells were transfected with expression plasmids encoding LacZ (control), HDJ-1, EF-1α, or HSP84. After 24 hr the cells were replated to the 48-well plates at a density of 5 × 104/well and then differentiated by the addition of 5 m
m
db-cAMP (light gray bars), or the cells were differentiated and induced tNhtt-150Q-GFP by 5 m
m
db-cAMP plus 1 μ
m
ponasterone A (dark gray bars). Cell viability on the fourth day of induction was measured by MTT assay and presented as a percentage of differentiated-only LacZ-overexpressing control (indicated as_Ctrl_). Values are the means ± SEM;n = 12. *p < 0.01, compared by Student's t test with a ponasterone A-treated LacZ-overexpressing experiment. b, Expressions of LacZ (lane 1), HDJ-1 (lane 2), EF-1α (lane 3), and HSP84 (lane 4) in the transfected HD150Q-28 cells were confirmed by Western blotting at 2 d after the treatment with db-cAMP and ponasterone A. Bands were detected with anti-v5 antibody. c, Western blot analysis to check the effect of transient overexpression of HDJ-1, EF-1α, and HSP84 on the expression of tNhtt-polyQ-GFP from differentiated and induced HD150Q-28 and HD16Q-23 cell lines. The expression level of tNhtt-polyQ-GFP was detected by anti-N-terminal huntingtin antibody, showing no differences among those transfected cells.
Fig. 9.
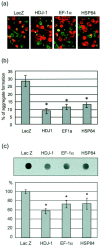
Effect of EF-1α and HSP84 on aggregate formation. a, HD150Q-28 cells overexpressing LacZ, HDJ-1, EF-1α, and HSP84, followed by differentiation and induction with 5 m
m
db-cAMP plus 0.1 μ
m
ponasterone A, were probed with anti-v5 antibody and stained with Alexa Fluor 546-labeled secondary antibody. The panels show the typical immunofluorescent images. Red, green, and_yellow_ indicate v5, tNhtt-150Q-GFP, and the coexistence of both molecules, respectively. b, The frequency of aggregate formation in the v5-positive cells was measured by counting the numbers of aggregate-positive cells. The data are presented as the percentage of aggregate and v5 double-positive cells in total v5-positive cells. Values are the means ± SEM;n = 10. *p < 0.01, compared by Student's t test with a control experiment that used LacZ-expressing cells. c, Filter retardation assay for comparing aggregate formation in control (_LacZ_-), HDJ-1-, EF-1α-, and HSP84-overexpressing cells. The aggregated form of tNhtt-150Q-GFP in the cells was trapped on the cellulose acetate membrane; the tNhtt-immunoreactive spots were detected (top), and the density was quantified (bottom) as described in Materials and Methods. The significant reduction of SDS insoluble aggregates was observed in HDJ-1-, EF-1α-, and HSP84-overexpressing cells. Values are the means ± SEM; n = 4. *p < 0.01, compared by Student's _t_test with a control experiment that used LacZ-expressing cells.
Similar articles
- Proteomics of polyglutamine aggregates.
Mitsui K, Doi H, Nukina N. Mitsui K, et al. Methods Enzymol. 2006;412:63-76. doi: 10.1016/S0076-6879(06)12005-4. Methods Enzymol. 2006. PMID: 17046652 Review. - Modulating huntingtin half-life alters polyglutamine-dependent aggregate formation and cell toxicity.
Kaytor MD, Wilkinson KD, Warren ST. Kaytor MD, et al. J Neurochem. 2004 May;89(4):962-73. doi: 10.1111/j.1471-4159.2004.02376.x. J Neurochem. 2004. PMID: 15140195 - Ultrastructure of nuclear aggregates formed by expressing an expanded polyglutamine.
Hazeki N, Tsukamoto T, Yazawa I, Koyama M, Hattori S, Someki I, Iwatsubo T, Nakamura K, Goto J, Kanazawa I. Hazeki N, et al. Biochem Biophys Res Commun. 2002 Jun 7;294(2):429-40. doi: 10.1016/S0006-291X(02)00498-9. Biochem Biophys Res Commun. 2002. PMID: 12051730 - Evidence for both the nucleus and cytoplasm as subcellular sites of pathogenesis in Huntington's disease in cell culture and in transgenic mice expressing mutant huntingtin.
Hackam AS, Hodgson JG, Singaraja R, Zhang T, Gan L, Gutekunst CA, Hersch SM, Hayden MR. Hackam AS, et al. Philos Trans R Soc Lond B Biol Sci. 1999 Jun 29;354(1386):1047-55. doi: 10.1098/rstb.1999.0457. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10434304 Free PMC article. Review.
Cited by
- Neurodegeneration caused by polyglutamine expansion is regulated by P-glycoprotein in Drosophila melanogaster.
Yadav S, Tapadia MG. Yadav S, et al. Genetics. 2013 Nov;195(3):857-70. doi: 10.1534/genetics.113.155077. Epub 2013 Sep 13. Genetics. 2013. PMID: 24037265 Free PMC article. - Molecular chaperone Hsp90 stabilizes Pih1/Nop17 to maintain R2TP complex activity that regulates snoRNA accumulation.
Zhao R, Kakihara Y, Gribun A, Huen J, Yang G, Khanna M, Costanzo M, Brost RL, Boone C, Hughes TR, Yip CM, Houry WA. Zhao R, et al. J Cell Biol. 2008 Feb 11;180(3):563-78. doi: 10.1083/jcb.200709061. J Cell Biol. 2008. PMID: 18268103 Free PMC article. - Sex-dependent impaired locomotion and motor coordination in the HdhQ200/200 mouse model of Huntington's Disease.
Cao JK, Viray K, Zweifel L, Stella N. Cao JK, et al. Neurobiol Dis. 2019 Dec;132:104607. doi: 10.1016/j.nbd.2019.104607. Epub 2019 Sep 6. Neurobiol Dis. 2019. PMID: 31499139 Free PMC article. - Role of heat shock proteins during polyglutamine neurodegeneration: mechanisms and hypothesis.
Wyttenbach A. Wyttenbach A. J Mol Neurosci. 2004;23(1-2):69-96. doi: 10.1385/JMN:23:1-2:069. J Mol Neurosci. 2004. PMID: 15126694 Review.
References
- Chai Y, Koppenhafer SL, Shoesmith SJ, Perez MK, Paulson HL. Evidence for proteasome involvement in polyglutamine disease: localization to nuclear inclusions in SCA3/MJD and suppression of polyglutamine aggregation in vitro. Hum Mol Genet. 1999;8:673–682. - PubMed
- Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, Patterson C. The co-chaperone CHIP regulates protein triage decisions mediated by heat shock proteins. Nat Cell Biol. 2001;3:93–96. - PubMed
- Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G. The 90 kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79:129–168. - PubMed
- Cummings CJ, Mancini MA, Antalffy B, DeFranco DB, Orr HT, Zoghbi HY. Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nat Genet. 1998;19:148–154. - PubMed
- Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous