Protein kinase C-dependent phosphorylation of synaptosome-associated protein of 25 kDa at Ser187 potentiates vesicle recruitment - PubMed (original) (raw)
Protein kinase C-dependent phosphorylation of synaptosome-associated protein of 25 kDa at Ser187 potentiates vesicle recruitment
Gábor Nagy et al. J Neurosci. 2002.
Abstract
Activation of protein kinase C (PKC) constitutes a key event in the upregulation of secretory strength in neurons and neurosecretory cells during extensive stimulation, presumably by speeding up vesicle supply. However, the molecular targets and their mode of action remain elusive. We studied the only PKC-dependent phosphorylation site in the neuronal soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex, Ser(187), in synaptosome-associated protein of 25 kDa (SNAP-25). This phosphorylation site is located within the negatively charged C-terminal end of SNAP-25, which has been shown to be of critical importance in calcium-triggered exocytosis. We combined mutational studies that used overexpression in chromaffin cells with capacitance measurements and flash photolysis of caged calcium, allowing for high time resolution during both the stimulation and measurement of exocytosis. Overexpression of mutants simulating the phosphorylated form of Ser(187) accelerated vesicle recruitment after the emptying of the releasable vesicle pools. Overexpression of mutants simulating the nonphosphorylated form, or block of PKC, impaired the refilling of the vesicle pools to similar extents. Biochemical studies verified the phosphorylation of a subpopulation of SNAP-25 after elevation of intracellular calcium concentrations. Some of the mutations led to a moderately decreased fast exocytotic burst component, which did not seem to be associated with the phosphorylation state of SNAP-25. Thus the C terminus of SNAP-25 plays a role for both fast exocytosis triggering and vesicle recruitment, and the latter process is regulated by PKC-dependent phosphorylation.
Figures
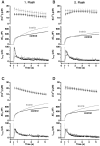
Fig. 1.
Mutants mimicking the phosphorylated state of Ser187 (aspartate and glutamate substitution) lead to an increased sustained component of secretion. A, Averaged calcium concentration (top), capacitance (middle), and amperometric responses (bottom) after a step-like elevation of [Ca2+]i induced by flash photolysis of a calcium cage (flash at arrow). The capacitance trace displays a burst-like increase within the first 1 sec after the flash, which is followed by a slower sustained phase of secretion, representing vesicle recruitment (priming) and consecutive fusion. Overexpression of S187D mutant (gray trace;n = 22) led to an increased sustained phase of the secretion compared with the nontransfected control cells (black trace; n = 18). B, The corresponding response evoked by the second flash stimulation 80 sec later shows a similar increase in the sustained component of secretion.C, Overexpression of S187E (gray trace; n = 21) also led to an increase in the sustained phase of exocytosis caused by the first flash compared with control cells (black trace; n = 27). D, The corresponding response evoked by the second flash stimulation 80 sec later shows a similar increase in the sustained component of secretion.
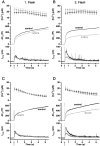
Fig. 2.
Mutants mimicking the nonphosphorylated state of Ser187 (alanine and cysteine substitution) reduce the responses evoked by the second flash (flash at_arrow_). A, Overexpression of S187A mutant (gray trace; n = 18) slightly decreased the overall secretory response to the first flash (flash at_arrow_) compared with the control group (black trace; n = 18). B, A substantial reduction in the second flash-evoked secretion was observed for the S187A mutant, mainly because of a decrease in the exocytotic burst phase. C, Overexpression of S187C (gray trace; n = 19) did not affect the response to the first flash compared with the control cells (black trace; n = 19).D, The response to the second flash-evoked secretion was diminished in the S187C mutant-expressing cells because of a decrease in the exocytotic burst phase.
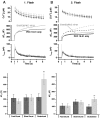
Fig. 3.
Protein kinase C inhibitor peptide, but not SNAP-25 overexpression, reduces the response to the second flash.A, B, Overexpression of wild-type SNAP-25-GFP (gray trace; n = 22) did not affect the response to the first or second flash stimulation (flash at_arrow_) compared with control cells (black trace; n = 16). C, Inclusion of 10 μ
m
PKC inhibitor peptide (PKC 19–31; gray trace; n = 16) in the pipette had no effect on the secretory response evoked by the first flash compared with control cells (black trace; n = 14).D, PKC 19–31 inhibited the response evoked by the second flash because of a reduced burst phase of exocytosis.
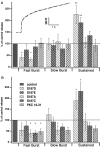
Fig. 4.
Analysis of the three kinetic components of secretion. A, The inset shows a typical secretory response to the flash in bovine chromaffin cells (gray trace) and, superimposed on it, a triple exponential fit that was used to determine the amplitudes and time constants of the kinetic components (black dotted line). The bar diagram shows the amplitude of each kinetic component of secretion, normalized to control values obtained from the same cell preparations. The time constants did not vary significantly between mutants and conditions and therefore are not displayed. Both of the phosphomimetic mutants (S187D, S187E) caused a statistically significant increase in the amplitude of the sustained component of the secretion evoked by the first flash. The substitution of Ser187 with aspartate (S187D) or alanine (S187A) significantly reduced the fast burst component of secretion. S187C as well as the inhibition of PKC (PKC 19–31) had no significant effects on the different kinetic components of secretion evoked by the first flash stimulation. B, Overexpression of nonphosphomimetic mutants (S187A, S187C) and inhibition of PKC (PKC 19–31) led to a depression of both the fast and the slow component of the exocytotic burst evoked by the second flash. The increase in the sustained component was still present with the two phosphomimetic mutants (S187D, S187E) as well as the depression of the fast burst component in the case of S187D. *p < 0.05; **p < 0.01. Data are displayed as mean ± SEM.
Fig. 5.
Overexpression of the SNAP-25 S187E mutant cannot prevent the reduction of the response to the second flash induced by PKC inhibition. A, Averaged calcium concentration (top), capacitance (middle), and amperometric responses (bottom) measured after the introduction of 10 μ
m
PKC inhibitor (PKC 19–31) into the cells through the pipette. Overexpression of S187E in the presence of PKC inhibition still led to an increase in the sustained phase of exocytosis evoked by the first flash (gray traces; n = 18) compared with the nontransfected cells (black traces;n = 15). The amplitudes of the three kinetic components are indicated at the bottom(black, nontransfected; gray, S187E). When the two conditions are compared, only the difference between the two sustained phases is statistically significant; *p < 0.05. B, In the secretory response caused by the second flash stimulation in the transfected cells (gray traces) and the nontransfected cells (black traces), both are reduced compared with the results without PKC inhibitor (compare with Fig.1_D_). Dashed capacitance traces indicate the expected secretion without PKC inhibitor (gray for mutants and black for control cells). At the bottom, a triple exponential fit reveals a tendency toward an increase in the sustained phase of the transfected cells even under these conditions; ∼p< 0.1. Data are displayed as mean ± SEM.
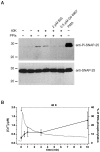
Fig. 6.
Phosphorylation of SNAP-25 at Ser187. A, Western blot analysis with an antibody against the Ser187-phosphorylated form of SNAP-25 (top). Control cells had very low amounts of phosphorylated SNAP-25. The extracellular application of 40 m
m
K+ together with 10 m
m
Ca2+ (40K) for 10 min led to the phosphorylation of a subpopulation of SNAP-25. Phosphorylation could be blocked by preincubation with 2 μ
m
BIS or 500 n
m
Gö 6976, indicating that phosphorylation was mediated by PKC. Adding 10 μ
m
calyculin A, 1 n
m
cypermethrin, and 1 μ
m
cyclosporine A (protein phosphatase inhibitors, PPIs) did not alter the amount of phosphorylated SNAP-25. Stimulation of the cells with 100 n
m
PMA for 20 min led to massive phosphorylation (last lane). The bottom panels show the detection of SNAP-25 with a monoclonal antibody against the N terminus of the protein to demonstrate loading of similar amounts of protein into each lane. Marker lines are shown in kilodaltons.B, The time course of the phosphorylation caused by the 40K solution was studied by quantitative Western blot analysis and displayed as a fraction of the PMA-induced phosphorylation in separate experiments (black symbols, right axis; error bars indicate mean ± SEM; n = 5–7). Depolarization caused an increase in phosphorylation of SNAP-25 within 30 sec and a further increase after 10 min. Also plotted is the time course of [Ca2+]i change in fura-2 AM-loaded cells (n = 8) exposed to the depolarizing solution (continuous gray curve, left axis).
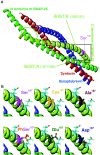
Fig. 7.
Structural arrangement of _in vivo_amino acids and mutants at position 187 in SNAP-25. A, An overall view of the core complex indicating the localization of Ser187 (modified after Sutton et al., 1998).B, The top row displays the structural configuration of Ser187 (top left) and its nonphosphorylated substitutes, cysteine (top middle) and alanine (top right), in higher magnification. The side chains of the neighboring negatively charged amino acids Glu183 and Glu194 in the C-terminal end also are indicated with light blue sticks in all panels. The polar oxygen and sulfur are displayed in light pink. The bottom row displays the in vivo phosphoserine (bottom left) and the two amino acids that were used to simulate it, glutamate (bottom middle) and aspartate (bottom right). The phosphoserine can adopt just one possible rotamer conformation in the complex. Although both glutamate and aspartate have more possible rotamers, glutamate can simulate phosphoserine satisfactorily, whereas aspartate most likely cannot (the negative oxygen is emphasized with red).
Similar articles
- Regulation of releasable vesicle pool sizes by protein kinase A-dependent phosphorylation of SNAP-25.
Nagy G, Reim K, Matti U, Brose N, Binz T, Rettig J, Neher E, Sørensen JB. Nagy G, et al. Neuron. 2004 Feb 5;41(3):417-29. doi: 10.1016/s0896-6273(04)00038-8. Neuron. 2004. PMID: 14766180 - Exocytotic mechanism studied by truncated and zero layer mutants of the C-terminus of SNAP-25.
Wei S, Xu T, Ashery U, Kollewe A, Matti U, Antonin W, Rettig J, Neher E. Wei S, et al. EMBO J. 2000 Mar 15;19(6):1279-89. doi: 10.1093/emboj/19.6.1279. EMBO J. 2000. PMID: 10716928 Free PMC article. - Early requirement for alpha-SNAP and NSF in the secretory cascade in chromaffin cells.
Xu T, Ashery U, Burgoyne RD, Neher E. Xu T, et al. EMBO J. 1999 Jun 15;18(12):3293-304. doi: 10.1093/emboj/18.12.3293. EMBO J. 1999. PMID: 10369670 Free PMC article. - Regulation of exocytosis by protein kinase C.
Morgan A, Burgoyne RD, Barclay JW, Craig TJ, Prescott GR, Ciufo LF, Evans GJ, Graham ME. Morgan A, et al. Biochem Soc Trans. 2005 Dec;33(Pt 6):1341-4. doi: 10.1042/BST0331341. Biochem Soc Trans. 2005. PMID: 16246114 Review. - Spatio-temporal regulation of neurotransmitter release by PKC; studies in adrenal chromaffin cells.
Kumakura K, Sasakawa N, Murayama N, Ohara-Imaizumi M. Kumakura K, et al. Crit Rev Neurobiol. 2004;16(1-2):173-9. doi: 10.1615/critrevneurobiol.v16.i12.180. Crit Rev Neurobiol. 2004. PMID: 15581412 Review.
Cited by
- TrkB signaling is correlated with muscular fatigue resistance and less vulnerability to neurodegeneration.
Just-Borràs L, Cilleros-Mañé V, Polishchuk A, Balanyà-Segura M, Tomàs M, Garcia N, Tomàs J, Lanuza MA. Just-Borràs L, et al. Front Mol Neurosci. 2022 Dec 22;15:1069940. doi: 10.3389/fnmol.2022.1069940. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36618825 Free PMC article. - Synaptic retrograde regulation of the PKA-induced SNAP-25 and Synapsin-1 phosphorylation.
Polishchuk A, Cilleros-Mañé V, Just-Borràs L, Balanyà-Segura M, Vandellòs Pont G, Silvera Simón C, Tomàs M, Garcia N, Tomàs J, Lanuza MA. Polishchuk A, et al. Cell Mol Biol Lett. 2023 Mar 3;28(1):17. doi: 10.1186/s11658-023-00431-2. Cell Mol Biol Lett. 2023. PMID: 36869288 Free PMC article. - Inhibition of cellular responses to insulin in a rat liver cell line. A role for PKC in insulin resistance.
Puljak L, Pagliassotti MJ, Wei Y, Qadri I, Parameswara V, Esser V, Fitz JG, Kilic G. Puljak L, et al. J Physiol. 2005 Mar 1;563(Pt 2):471-82. doi: 10.1113/jphysiol.2004.080333. Epub 2005 Jan 13. J Physiol. 2005. PMID: 15649984 Free PMC article. - The Impact of Kinases in Amyotrophic Lateral Sclerosis at the Neuromuscular Synapse: Insights into BDNF/TrkB and PKC Signaling.
Lanuza MA, Just-Borràs L, Hurtado E, Cilleros-Mañé V, Tomàs M, Garcia N, Tomàs J. Lanuza MA, et al. Cells. 2019 Dec 5;8(12):1578. doi: 10.3390/cells8121578. Cells. 2019. PMID: 31817487 Free PMC article. Review. - The SNAP-25 linker as an adaptation toward fast exocytosis.
Nagy G, Milosevic I, Mohrmann R, Wiederhold K, Walter AM, Sørensen JB. Nagy G, et al. Mol Biol Cell. 2008 Sep;19(9):3769-81. doi: 10.1091/mbc.e07-12-1218. Epub 2008 Jun 25. Mol Biol Cell. 2008. PMID: 18579690 Free PMC article.
References
- Ashery U, Betz A, Xu T, Brose N, Rettig J. An efficient method for infection of adrenal chromaffin cells using the Semliki Forest virus gene expression system. Eur J Cell Biol. 1999;78:525–532. - PubMed
- Augustin I, Rosenmund C, Südhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. - PubMed
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Südhof TC, Rettig J, Brose N. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources