Increased extracellular amyloid deposition and neurodegeneration in human amyloid precursor protein transgenic mice deficient in receptor-associated protein - PubMed (original) (raw)
Increased extracellular amyloid deposition and neurodegeneration in human amyloid precursor protein transgenic mice deficient in receptor-associated protein
Emily Van Uden et al. J Neurosci. 2002.
Abstract
The low-density lipoprotein receptor-related protein (LRP) is an abundant neuronal cell surface receptor that regulates amyloid beta-protein (Abeta) trafficking into the cell. Specifically, LRP binds secreted Abeta complexes and mediates its degradation. Previously, we have shown in vitro that the uptake of Abeta mediated by LRP is protective and that blocking this receptor significantly enhances neurotoxicity. To further characterize the effects of LRP and other lipoprotein receptors on Abeta deposition, an in vivo model of decreased LRP expression, receptor-associated protein (RAP)-deficient (RAP-/-) mice was crossed with human amyloid protein precursor transgenic (hAPP tg) mice, and plaque formation and neurodegeneration were analyzed. We found that, although the age of onset for plaque formation was the same in hAPP tg and hAPP tg/RAP-/- mice, the amount of amyloid deposited doubled in the hAPP tg/RAP-/- background. Moreover, these mice displayed increased neuronal damage and astrogliosis. Together, these results further support the contention that LRP and other lipoprotein receptors might be neuroprotective against Abeta toxicity and that this receptor might play an integral role in Abeta clearance.
Figures
Fig. 1.
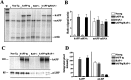
Characterization of APP and LRP expression in hAPP tg/RAP−/− mice. A, Ribonuclease protection assay. A total of four mice per group was analyzed. Representative autoradiogram for mAPP and hAPP is shown. The_leftmost_ lane represents the undigested (U) radiolabeled probes; the other_lanes_ contain the same riboprobes plus brain RNA samples digested with RNases. Protected mRNAs are indicated on the_right_. The hAPP, mAPP, and actin mRNA bands were detected as fragments of 230, 180, and 85 nt, respectively.B, Analysis of levels of hAPP and mAPP mRNA expression in tg mice; results are expressed as a ratio of APP to actin. Error bars are mean ± SEM. C, Western blot analysis with antibodies against hAPP and LRP. The hAPP-specific antibody identified a broad band at an approximate molecular weight (MW) of 110 kDa. The specific antibody against the C-terminal region of LRP detected a band at an approximate MW of 85 kDa.D, Analysis of hAPP and LRP immunoreactivity; results are expressed as integrated pixel intensity. Error bars are mean ± SEM.
Fig. 2.
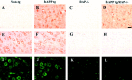
Immunocytochemical analysis of hAPP, RAP, and LRP expression in hAPP tg/RAP−/− mice. A–D, hAPP immunoreactivity with the 8E5 monoclonal antibody; E–H, RAP immunoreactivity in the frontal cortex of 18-month-old mice;I–L, LRP immunoreactivity in the frontal cortex of 18-month-old mice. A, No hAPP immunoreactivity was observed in non-tg mice. B, hAPP tg mice displayed immunostaining of a subset of pyramidal neurons in the neocortex.C, No hAPP immunoreactivity was observed in RAP−/− mice. D, hAPP tg/RAP−/− tg mice hAPP-immunoreactive pyramidal neurons in the neocortex. E, Non-tg;F, hAPP tg mice showed extensive immunostaining of pyramidal neurons in the neocortex. In the RAP−/− (G) and hAPP tg/RAP−/− (H), no RAP immunoreactivity was observed. Non-tg (I) and hAPP tg (J) mice showed extensive LRP immunostaining of pyramidal neurons in the neocortex. In the RAP−/− (G) and hAPP tg/RAP−/− (H), no LRP levels of LRP immunoreactivity were decreased. Scale bar, 25 μm.
Fig. 3.
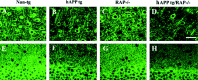
Patterns of Aβ immunoreactivity in hAPP tg/RAP−/−. A–D, Aβ immunoreactivity in the neocortex; E–H, low-power view (60×) of the hippocampal dentate gyrus; I–L, higher-power view (200×) of the dentate gyrus; M, P, GFAP immunoreactivity in the hippocampus. All images are from 18-month-old mice. A, No Aβ deposits were observed in non-tg controls. B, Mature and diffuse amyloid plaques in hAPP tg/RAP tg mice. C, No Aβ deposits were observed in RAP−/− mice. D, hAPP tg/RAP−/− mice displayed increased Aβ immunoreactive plaques. Scale bar, 20 μm.E, I, No Aβ deposits were observed in the molecular layer of the dentate in non-tg controls.F, J, Abundant diffuse amyloid plaques in hAPP tg mice. G, K, No Aβ deposits were observed in RAP−/− mice. H, L, hAPP tg/RAP−/− mice displayed increased Aβ deposits in the molecular layer of the dentate. Scale bar, 60 μm. M, Mild astrogliosis in non-tg mice. N, Increased astroglial reaction in hAPP mice. O, Mild astroglial immunostaining in RAP−/− mice. P, Enhanced astroglial reaction in hAPP tg/RAP−/− mice. Scale bar, 30 μm.
Fig. 4.
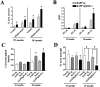
Quantitative analysis of amyloid production and neurodegeneration. A, Determination of percentage area of the neuropil occupied by Aβ immunoreactive deposits in the frontal cortex and hippocampus. B, Determination of Aβ levels in the hippocampus by ELISA in 10- and 18-month-old mice. *p < 0.05, different by two-tailed unpaired_t_ test when compared with hAPP tg mice.C, Densitometrical analysis of GFAP immunoreactivity in the hippocampus using the Quantimet 570C in 10- and 18-month-old mice. *p < 0.05 different from non-tg control by one-way ANOVA post hoc Dunnet's test. D, Levels of MAP2-IR in the outer molecular layer of the dentate gyrus in 10- and 18-month-old mice. *p < 0.05, different by one-way ANOVA post hoc Dunnet's test.
Fig. 5.
Patterns of dendritic degeneration in hAPP tg/RAP−/−. Sections from 18-month-old mice were immunolabeled with an antibody against MAP2 and imaged with the laser scanning confocal microscope. A–D, Neocortex; E–H, molecular layer of the hippocampal dentate gyrus. Scale bar, 20 μm.
Similar articles
- Receptor-associated protein (RAP) plays a central role in modulating Abeta deposition in APP/PS1 transgenic mice.
Xu G, Karch C, Li N, Lin N, Fromholt D, Gonzales V, Borchelt DR. Xu G, et al. PLoS One. 2008 Sep 8;3(9):e3159. doi: 10.1371/journal.pone.0003159. PLoS One. 2008. PMID: 18776935 Free PMC article. - Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation.
Buttini M, Yu GQ, Shockley K, Huang Y, Jones B, Masliah E, Mallory M, Yeo T, Longo FM, Mucke L. Buttini M, et al. J Neurosci. 2002 Dec 15;22(24):10539-48. doi: 10.1523/JNEUROSCI.22-24-10539.2002. J Neurosci. 2002. PMID: 12486146 Free PMC article. - Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer's disease.
Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, Ghetti B, Paul SM. Bales KR, et al. Proc Natl Acad Sci U S A. 1999 Dec 21;96(26):15233-8. doi: 10.1073/pnas.96.26.15233. Proc Natl Acad Sci U S A. 1999. PMID: 10611368 Free PMC article. - Abeta-degrading endopeptidase, neprilysin, in mouse brain: synaptic and axonal localization inversely correlating with Abeta pathology.
Fukami S, Watanabe K, Iwata N, Haraoka J, Lu B, Gerard NP, Gerard C, Fraser P, Westaway D, St George-Hyslop P, Saido TC. Fukami S, et al. Neurosci Res. 2002 May;43(1):39-56. doi: 10.1016/s0168-0102(02)00015-9. Neurosci Res. 2002. PMID: 12074840 - LRP in amyloid-beta production and metabolism.
Bu G, Cam J, Zerbinatti C. Bu G, et al. Ann N Y Acad Sci. 2006 Nov;1086:35-53. doi: 10.1196/annals.1377.005. Ann N Y Acad Sci. 2006. PMID: 17185504 Review.
Cited by
- A brain proteomic signature of incipient Alzheimer's disease in young APOE ε4 carriers identifies novel drug targets.
Roberts JA, Varma VR, An Y, Varma S, Candia J, Fantoni G, Tiwari V, Anerillas C, Williamson A, Saito A, Loeffler T, Schilcher I, Moaddel R, Khadeer M, Lovett J, Tanaka T, Pletnikova O, Troncoso JC, Bennett DA, Albert MS, Yu K, Niu M, Haroutunian V, Zhang B, Peng J, Croteau DL, Resnick SM, Gorospe M, Bohr VA, Ferrucci L, Thambisetty M. Roberts JA, et al. Sci Adv. 2021 Nov 12;7(46):eabi8178. doi: 10.1126/sciadv.abi8178. Epub 2021 Nov 10. Sci Adv. 2021. PMID: 34757788 Free PMC article. - Alzheimer's amyloid-β and tau protein accumulation is associated with decreased expression of the LDL receptor-associated protein in human brain tissue.
Shepherd CE, Affleck AJ, Bahar AY, Carew-Jones F, Gregory G, Small DH, Halliday GM. Shepherd CE, et al. Brain Behav. 2020 Jul;10(7):e01672. doi: 10.1002/brb3.1672. Epub 2020 Jun 2. Brain Behav. 2020. PMID: 32484608 Free PMC article. - A Role of Low-Density Lipoprotein Receptor-Related Protein 4 (LRP4) in Astrocytic Aβ Clearance.
Zhang H, Chen W, Tan Z, Zhang L, Dong Z, Cui W, Zhao K, Wang H, Jing H, Cao R, Kim C, Safar JG, Xiong WC, Mei L. Zhang H, et al. J Neurosci. 2020 Jul 8;40(28):5347-5361. doi: 10.1523/JNEUROSCI.0250-20.2020. Epub 2020 May 26. J Neurosci. 2020. PMID: 32457076 Free PMC article. - Low-dose pioglitazone can ameliorate learning and memory impairment in a mouse model of dementia by increasing LRP1 expression in the hippocampus.
Seok H, Lee M, Shin E, Yun MR, Lee YH, Moon JH, Kim E, Lee PH, Lee BW, Kang ES, Lee HC, Cha BS. Seok H, et al. Sci Rep. 2019 Mar 13;9(1):4414. doi: 10.1038/s41598-019-40736-x. Sci Rep. 2019. PMID: 30867485 Free PMC article. - Elimination of substances from the brain parenchyma: efflux via perivascular pathways and via the blood-brain barrier.
Hladky SB, Barrand MA. Hladky SB, et al. Fluids Barriers CNS. 2018 Oct 19;15(1):30. doi: 10.1186/s12987-018-0113-6. Fluids Barriers CNS. 2018. PMID: 30340614 Free PMC article. Review.
References
- Bales K, Verina T, Dodel R, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone E, Little S, Cummins D, Piccardo P, Ghetti B, Paul S. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat Genet. 1997;17:263–264. - PubMed
- Blacker D, Wilcox MA, Laird NM, Rodes L, Horvath SM, Go RC, Perry R, Watson B, Jr, Bassett SS, McInnis MG, Albert MS, Hyman BT, Tanzi RE. Alpha-2 macroglobulin is genetically associated with Alzheimer disease. Nat Genet. 1998;19:357–360. - PubMed
- Borth W. Alpha 2-macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 1992;6:3345–3353. - PubMed
- Bu G. Receptor-associated protein: a specialized chaperone and antagonist for members of the LDL receptor gene family. Curr Opin Lipidol. 1998;9:149–155. - PubMed
- Bu G. The roles of receptor-associated protein (RAP) as a molecular chaperone for members of the LDL receptor family. Int Rev Cytol. 2001;209:79–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG018440/AG/NIA NIH HHS/United States
- AG18440/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- AG5131/AG/NIA NIH HHS/United States
- R37 AG018440/AG/NIA NIH HHS/United States
- AG10689/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous