Differential effects of direct and indirect dopamine agonists on prepulse inhibition: a study in D1 and D2 receptor knock-out mice - PubMed (original) (raw)
Differential effects of direct and indirect dopamine agonists on prepulse inhibition: a study in D1 and D2 receptor knock-out mice
Rebecca J Ralph-Williams et al. J Neurosci. 2002.
Abstract
Stimulation of the dopamine (DA) system disrupts prepulse inhibition (PPI) of the acoustic startle response. On the basis of rat studies, it appeared that DA D2 receptors (D2Rs) rather than D1 receptors (D1Rs) regulate PPI, albeit possibly in synergism with D1Rs. To characterize the DA receptor modulation of PPI in another species, we tested DA D1R and D2R mutant mice with direct and indirect DA agonists and with the glutamate receptor antagonist, dizocilpine (MK-801). Neither the mixed D1/D2 agonist apomorphine (5 mg/kg) nor the more selective D1-like agonist SKF82958 (0.3 mg/kg) altered PPI in D1R knock-out mice, although both compounds disrupted PPI in D2R mutant and wild-type mice, suggesting that the D1R alone might modulate PPI in mice. However, amphetamine (10 mg/kg) significantly lowered PPI in each genotype of D1R mice, suggesting that the D1R is not necessary for the PPI-disruptive effect of the indirect agonist in mice. As reported previously, amphetamine (10 mg/kg) failed to disrupt PPI in D2R knock-out mice, supporting a unique role of the D2R in the modulation of PPI. Dizocilpine (0.3 mg/kg) induced similar PPI deficits in D1R and D2R mutant mice, confirming that the influences of the NMDA receptor on PPI are independent of D1Rs and D2Rs in rodents. Thus, both D1Rs and D2Rs modulate aspects of PPI in mice in a manner that differs from dopaminergic modulation in rats. These findings emphasize that further cross-species comparisons of the pharmacology of PPI are essential to understand the relevance of rodent PPI studies to the deficits in PPI observed in patients with schizophrenia.
Figures
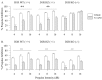
Fig. 1.
PPI levels in D1R and D2R mice after pretreatment with vehicle (0.1% ascorbic acid; open bars) or apomorphine (APO) (5.0 mg/kg; hatched bars). A, The mixed D1/D2 agonist apomorphine decreased PPI in both the D1R wild-type (WT, +/+) and heterozygous (HZ, +/−) mice but was ineffective in the D1R knock-out (KO, −/−) mice. **p < 0.01 compared with vehicle control.B, Apomorphine disrupted PPI in all D2R mutant mice, regardless of genotype (overall drug effect,### p < 0.001). Error bars indicate mean % PPI ± SEM.
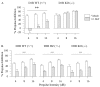
Fig. 2.
PPI levels in D1R and D2R mutant mice with vehicle control (water; open bars) or SKF82958 (SKF) (0.3 mg/kg; hatched bars).A, The D1-like agonist SKF82958 produced deficits in PPI in the D1R wild-type (WT) (+/+) mice but failed to produce deficits in the D1R knock-out (KO) (−/−) mice.HZ, Heterozygous. **p < 0.01 compared with vehicle control. B, SKF82958 significantly reduced PPI in each of the D2R genotypes (overall drug effect,### p < 0.001). Error bars indicate mean % PPI ± SEM.
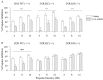
Fig. 3.
PPI levels for D1R and D2R mutant mice with vehicle control (water; open bars) or amphetamine (AMPH) (10 mg/kg; hatched bars).A, All D1R mice treated with the indirect DA agonist amphetamine displayed significant reductions in PPI compared with vehicle-treated mice (overall drug effect,### p < 0.001). B,Post hoc ANOVAs revealed that amphetamine significantly reduced PPI at the 4, 8, and 16 dB prepulse intensities in D2R wild-type (WT) (+/+) mice (**p < 0.01 compared with vehicle control) but had no significant effect in the heterozygous (HZ) (+/−) or knock-out (KO) (−/−) mice. Error bars indicate mean % PPI ± SEM.
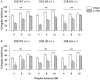
Fig. 4.
PPI levels in D1R and D2R mice after pretreatment with vehicle control (water; open bars) or dizocilpine (DIZ) (0.3 mg/kg; hatched bars). Regardless of genotype, the noncompetitive NMDA receptor antagonist dizocilpine significantly disrupted PPI in the D1R (A) and D2R (B) wild-type (WT) (+/+), heterozygous (HZ) (+/−), and knock-out (KO) (−/−) mice (overall drug effect,### p < 0.001). Error bars indicate mean % PPI ± SEM.
Similar articles
- Dopamine D1 rather than D2 receptor agonists disrupt prepulse inhibition of startle in mice.
Ralph-Williams RJ, Lehmann-Masten V, Geyer MA. Ralph-Williams RJ, et al. Neuropsychopharmacology. 2003 Jan;28(1):108-18. doi: 10.1038/sj.npp.1300017. Neuropsychopharmacology. 2003. PMID: 12496946 - Contributions of dopamine D1, D2, and D3 receptor subtypes to the disruptive effects of cocaine on prepulse inhibition in mice.
Doherty JM, Masten VL, Powell SB, Ralph RJ, Klamer D, Low MJ, Geyer MA. Doherty JM, et al. Neuropsychopharmacology. 2008 Oct;33(11):2648-56. doi: 10.1038/sj.npp.1301657. Epub 2007 Dec 12. Neuropsychopharmacology. 2008. PMID: 18075489 - Psychotropic drug-induced locomotor hyperactivity and prepulse inhibition regulation in male and female aromatase knockout (ArKO) mice: role of dopamine D1 and D2 receptors and dopamine transporters.
Chavez C, Gogos A, Jones ME, van den Buuse M. Chavez C, et al. Psychopharmacology (Berl). 2009 Oct;206(2):267-79. doi: 10.1007/s00213-009-1604-6. Epub 2009 Jul 14. Psychopharmacology (Berl). 2009. PMID: 19597801 - Novel Dopamine Therapeutics for Cognitive Deficits in Schizophrenia.
Arnsten AF, Girgis RR, Gray DL, Mailman RB. Arnsten AF, et al. Biol Psychiatry. 2017 Jan 1;81(1):67-77. doi: 10.1016/j.biopsych.2015.12.028. Epub 2016 Jan 18. Biol Psychiatry. 2017. PMID: 26946382 Free PMC article. Review. - The role of intracerebral dopamine D1 and D2 receptors in sleep-wake cycles and general anesthesia.
Zhang J, Li J, Liu CX, Gui H, Yuan CD, Zhang Y. Zhang J, et al. Ibrain. 2022 Feb 21;8(1):48-54. doi: 10.1002/ibra.12024. eCollection 2022 Spring. Ibrain. 2022. PMID: 37786416 Free PMC article. Review.
Cited by
- Rolipram: a specific phosphodiesterase 4 inhibitor with potential antipsychotic activity.
Kanes SJ, Tokarczyk J, Siegel SJ, Bilker W, Abel T, Kelly MP. Kanes SJ, et al. Neuroscience. 2007 Jan 5;144(1):239-46. doi: 10.1016/j.neuroscience.2006.09.026. Epub 2006 Nov 1. Neuroscience. 2007. PMID: 17081698 Free PMC article. - D-amphetamine and antipsychotic drug effects on latent inhibition in mice lacking dopamine D2 receptors.
Bay-Richter C, O'Callaghan MJ, Mathur N, O'Tuathaigh CM, Heery DM, Fone KC, Waddington JL, Moran PM. Bay-Richter C, et al. Neuropsychopharmacology. 2013 Jul;38(8):1512-20. doi: 10.1038/npp.2013.50. Epub 2013 Feb 19. Neuropsychopharmacology. 2013. PMID: 23422792 Free PMC article. - Baclofen reverses the reduction in prepulse inhibition of the acoustic startle response induced by dizocilpine, but not by apomorphine.
Bortolato M, Frau R, Aru GN, Orrù M, Gessa GL. Bortolato M, et al. Psychopharmacology (Berl). 2004 Jan;171(3):322-30. doi: 10.1007/s00213-003-1589-5. Epub 2003 Sep 10. Psychopharmacology (Berl). 2004. PMID: 13680072 - Sensorimotor gating in mice is disrupted after AM404, an anandamide reuptake and degradation inhibitor.
Fernandez-Espejo E, Galan-Rodriguez B. Fernandez-Espejo E, et al. Psychopharmacology (Berl). 2004 Sep;175(2):220-4. doi: 10.1007/s00213-004-1851-5. Epub 2004 Apr 16. Psychopharmacology (Berl). 2004. PMID: 15088080 - Are cross-species measures of sensorimotor gating useful for the discovery of procognitive cotreatments for schizophrenia?
Geyer MA. Geyer MA. Dialogues Clin Neurosci. 2006;8(1):9-16. doi: 10.31887/DCNS.2006.8.1/mgeyer. Dialogues Clin Neurosci. 2006. PMID: 16640109 Free PMC article. Review.
References
- Bakshi VP, Swerdlow NR, Geyer MA. Clozapine antagonizes phencyclidine-induced deficits in sensorimotor gating of the startle response. J Pharmacol Exp Ther. 1994;271:787–794. - PubMed
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. - PubMed
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. - PubMed
- Bullock AE, Slobe BS, Vazquez V, Collins AC. Inbred mouse strains differ in the regulation of startle and prepulse inhibition of the startle response. Behav Neurosci. 1997;111:1353–1360. - PubMed
- Curzon P, Decker MW. Effects of phencyclidine (PCP) and (+)MK-801 on sensorimotor gating in CD-1 mice. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:129–146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials