A secreted soluble form of ApoE receptor 2 acts as a dominant-negative receptor and inhibits Reelin signaling - PubMed (original) (raw)
A secreted soluble form of ApoE receptor 2 acts as a dominant-negative receptor and inhibits Reelin signaling
Stefanie Koch et al. EMBO J. 2002.
Abstract
Specialized neurons throughout the developing central nervous system secrete Reelin, which binds to ApoE receptor 2 (ApoER2) and very low density lipoprotein receptor (VLDLR), triggering a signal cascade that guides neurons to their correct position. Binding of Reelin to ApoER2 and VLDLR induces phosphorylation of Dab1, which binds to the intracellular domains of both receptors. Due to differential splicing, several isoforms of ApoER2 differing in their ligand-binding and intracellular domains exist. One isoform harbors four binding repeats plus an adjacent short 13 amino acid insertion containing a furin cleavage site. It is not known whether furin processing of this ApoER2 variant actually takes place and, if so, whether the produced fragment is secreted. Here we demonstrate that cleavage of this ApoER2 variant does indeed take place, and that the resulting receptor fragment consisting of the entire ligand-binding domain is secreted as soluble polypeptide. This receptor fragment inhibits Reelin signaling in primary neurons, indicating that it can act in a dominant-negative fashion in the regulation of Reelin signaling during embryonic brain development.
Figures
Fig. 1. Western blot analysis of cell extracts and supernatants of 293 cells expressing distinct ApoER2 variants. (A) Cartoon of the ApoER2 variants expressed in 293 cells and the recombinant soluble receptor fragment produced in E.coli. Numbered circles represent distinct ligand-binding repeats. F represents the domain carrying the furin cleavage site. A, B and C describe the cysteine-rich repeats of the EGF-homology domain. TM marks the transmembrane domain. MBP, maltose-binding protein; His, His6 tag. (B) 293 cells were transfected with ApoER2Δ4-6,8-F (lane 1), ApoER2Δ4-6 (lane 2) or empty vector (lane 3). Ten micrograms of the corresponding cell extracts were separated on a 10% SDS–PAGE gel under reducing conditions and transferred to a nitrocellulose membrane. ApoER2 was detected by western blotting using an antibody recognizing the first ligand-binding repeat (220). Binding of the primary antibody was visualized with HRP–goat anti-rabbit and a chemiluminescence system. (C) As (B), but western blotting was performed using an antibody against the cytoplasmic domain of the receptor (20). (D) 293 cells were transfected with ApoER2Δ4-6,8-F (lanes 1 and 4), ApoER2Δ4-6 (lane 2) or empty vector (lanes 3 and 5). The respective cell culture supernatants (1.5 ml) were incubated with 50 µl of RAP–Sepharose beads (lanes 1–3) or directly precipitated with TCA (lanes 4 and 5). The precipitated material was separated on a 15% SDS–PAGE gel under reducing (lanes 1–3) or non-reducing (lanes 4 and 5) conditions. For detection of the receptor fragment, Ab 220 was used in a western blot as described for (B). (E) 293 cells were transfected with ApoER2Δ4-6,8-F (lanes 1 and 2), ApoER2Δ4-6 (lanes 3 and 4) or empty vector (lanes 5 and 6) and incubated in the presence (+) or absence (–) of the furin inhibitor decanoyl-RVKR-chloromethylketone. The respective cell culture supernatants were used for detection of the soluble receptor fragment as described for (D).
Fig. 2. Recombinant ApoER21–3,7–MBP/His binds Reelin. (A) Five micrograms (lanes 1 and 2) or 0.5 µg (lanes 3–5) of recombinant ApoER21–3,7–MBP/His were separated on a 10% SDS–PAGE gel under reducing (lane 1) or non-reducing conditions (lanes 2–5). After transfer to a nitrocellulose membrane, lanes 1 and 2 were stained with Ponceau S, lanes 3 and 4 were processed for western blot analysis using the antibody against the first ligand-binding repeat of ApoER2 (220) (lane 3) or anti-MBP (lane 4), respectively. Lane 5 was incubated with Reelin-containing 293 medium and binding of Reelin was detected by western blotting using the antibody G10. Bound G10 was visualized with HRP–goat anti-mouse (1:15 000) and a chemiluminescence system. (B) Ten microliters of Reelin-conditioned medium were subjected to SDS–PAGE on a 6% gel, followed by a western blot using G10 as described in (A).
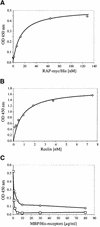
Fig. 3. (A and B) Binding of RAP-myc/His and Reelin to ApoER21–3,7–MBP/His. Ninety-six-well plates were coated with 10 µg/ml ApoER21–3,7–MBP/His. After incubation with the indicated amounts of either RAP-myc/His (A) or Reelin-containing 293T cell supernatant (B), bound RAP or Reelin was detected with anti-myc or anti-Reelin antibody and HRP-conjugated secondary antibody. (C) Ninety-six-well plates were coated with 10 µg/ml ApoER21–3,7–MBP/His (circles) or VLDLR1–8–MBP/His (squares) and incubated with Reelin (1 nM) in the presence of increasing amounts of recombinant ApoER21–3,7–MBP/His (0–80 µg/ml). Bound Reelin was detected with anti-Reelin antibody and HRP-conjugated secondary antibody.
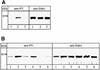
Fig. 4. Reelin-induced Dab1 phosphorylation is inhibited by the soluble ApoER2 fragment. Reelin-induced Dab1 phosphorylation was measured in primary mouse neurons after immunoprecipitation of Dab1 from cell lysates, followed by separation on an 8% SDS–PAGE gel and western blotting using anti-phosphotyrosine (PY) antibody and anti-Dab1 as described in Materials and methods. (A) Neurons were stimulated with control medium (lane 1), Reelin-conditioned medium (lane 2) or Reelin-conditioned medium in the presence of 40 µg/ml recombinant ApoER21–3,7–MBP/His (lane 3). Cells were processed for immunoprecipitation with anti-Dab1 and subsequent western blots were performed using anti-PY and anti-Dab1 as indicated. (B) Neurons were incubated with control medium (lane 1), Reelin-conditioned medium (lane 2), Reelin-conditioned medium in the presence of medium from 293 cells expressing ApoER2Δ4-6,8-F (lane 3) or in the presence of supernatant from 293 cells transfected with the empty plasmid (lane 4), medium from 293 cells expressing ApoER2Δ4-6,8-F (lane 5) or the empty plasmid (lane 6). Immunoprecipitation and western blot analysis as in (A).
Fig. 5. Analysis of splice variants of ApoER2 and detection of soluble ApoER2 in primary mouse neuronal cultures. (A) mRNA from embryonic brain from wild-type mice (lane 1), reeler mice (lane 3) and primary neuronal cultures (E15–16) (lane 2) was used for cDNA synthesis with reverse transcriptase and the resulting cDNA was used for PCR amplification as described in Materials and methods. Amplified products were separated on a 1.5% agarose gel. (B) Embryonic brains (E15–16) were dissected and the cerebrum (lane 1), the cerebellum (lane 2) and the olfactory bulbs (lane 3) were used for cDNA synthesis and PCR was performed as in (A). (C) Supernatant from a primary neuronal culture (E15–16) was immunoprecipitated with anti-Reelin antibody (G10). Immunocomplexes were precipitated with a mixture of protein A– and protein G–Sepharose and resolved on a 4.5–18% gradient polyacrylamide gel and transferred to nitrocellulose as described in Materials and methods. The soluble ApoER2 fragment was detected by western blotting using an antibody against the first ligand-binding repeat (220). Binding of the primary antibody was visualized with HRP–goat anti-rabbit (1:10 000) and a chemiluminescence system.
Fig. 6. Primary neurons in culture express and secrete Reelin. Primary mouse neuronal cultures (E15–16) were stained for the neuronal marker class III β-tubulin (TUJ1; red) and for Reelin (G10; green) as described in Materials and methods. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Roche). Bar: 30 µm. Inset: western blot analysis for the presence of Reelin in conditioned medium of primary mouse neurons. Supernatant (0.2 µl) from 293T cells expressing Reelin (lane A) and supernatant (15 µl) of a primary mouse E15–16 neuronal culture (lane B) were resolved on a 6% SDS–PAGE gel and tested for the presence of Reelin by western blotting using G10 as described in Materials and methods.
Similar articles
- Binding of purified Reelin to ApoER2 and VLDLR mediates tyrosine phosphorylation of Disabled-1.
Benhayon D, Magdaleno S, Curran T. Benhayon D, et al. Brain Res Mol Brain Res. 2003 Apr 10;112(1-2):33-45. doi: 10.1016/s0169-328x(03)00032-9. Brain Res Mol Brain Res. 2003. PMID: 12670700 - Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation.
Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Hiesberger T, et al. Neuron. 1999 Oct;24(2):481-9. doi: 10.1016/s0896-6273(00)80861-2. Neuron. 1999. PMID: 10571241 - Splicing variations in the ligand-binding domain of ApoER2 results in functional differences in the binding properties to Reelin.
Hibi T, Mizutani M, Baba A, Hattori M. Hibi T, et al. Neurosci Res. 2009 Apr;63(4):251-8. doi: 10.1016/j.neures.2008.12.009. Epub 2009 Jan 9. Neurosci Res. 2009. PMID: 19167437 - The reelin signaling pathway: some recent developments.
Jossin Y, Bar I, Ignatova N, Tissir F, De Rouvroit CL, Goffinet AM. Jossin Y, et al. Cereb Cortex. 2003 Jun;13(6):627-33. doi: 10.1093/cercor/13.6.627. Cereb Cortex. 2003. PMID: 12764038 Review. - [Corticohistogenesis and Reelin signal cascade].
Ogawa M. Ogawa M. Nihon Shinkei Seishin Yakurigaku Zasshi. 2000 Oct;20(4):169-74. Nihon Shinkei Seishin Yakurigaku Zasshi. 2000. PMID: 11215402 Review. Japanese.
Cited by
- Structure of a receptor-binding fragment of reelin and mutational analysis reveal a recognition mechanism similar to endocytic receptors.
Yasui N, Nogi T, Kitao T, Nakano Y, Hattori M, Takagi J. Yasui N, et al. Proc Natl Acad Sci U S A. 2007 Jun 12;104(24):9988-93. doi: 10.1073/pnas.0700438104. Epub 2007 Jun 4. Proc Natl Acad Sci U S A. 2007. PMID: 17548821 Free PMC article. - Receptor clustering is involved in Reelin signaling.
Strasser V, Fasching D, Hauser C, Mayer H, Bock HH, Hiesberger T, Herz J, Weeber EJ, Sweatt JD, Pramatarova A, Howell B, Schneider WJ, Nimpf J. Strasser V, et al. Mol Cell Biol. 2004 Feb;24(3):1378-86. doi: 10.1128/MCB.24.3.1378-1386.2004. Mol Cell Biol. 2004. PMID: 14729980 Free PMC article. - Human APOER2 Isoforms Have Differential Cleavage Events and Synaptic Properties.
Omuro KC, Gallo CM, Scrandis L, Ho A, Beffert U. Omuro KC, et al. J Neurosci. 2022 May 18;42(20):4054-4068. doi: 10.1523/JNEUROSCI.1800-21.2022. Epub 2022 Apr 12. J Neurosci. 2022. PMID: 35414534 Free PMC article. - Thrombospondin-1 binds to ApoER2 and VLDL receptor and functions in postnatal neuronal migration.
Blake SM, Strasser V, Andrade N, Duit S, Hofbauer R, Schneider WJ, Nimpf J. Blake SM, et al. EMBO J. 2008 Nov 19;27(22):3069-80. doi: 10.1038/emboj.2008.223. Epub 2008 Oct 23. EMBO J. 2008. PMID: 18946489 Free PMC article. - The ApoE receptors Vldlr and Apoer2 in central nervous system function and disease.
Lane-Donovan C, Herz J. Lane-Donovan C, et al. J Lipid Res. 2017 Jun;58(6):1036-1043. doi: 10.1194/jlr.R075507. Epub 2017 Mar 14. J Lipid Res. 2017. PMID: 28292942 Free PMC article. Review.
References
- Battey F.D., Gåfvels,M.E., FitzGerald,D.J., Argraves,W.S., Chappell, D.A., Strauss,J.F.,III and Strickland,D.K. (1994) The 39-kDa receptor-associated protein regulates ligand binding by the very low density lipoprotein receptor. J. Biol. Chem., 269, 23268–23273. - PubMed
- Brandes C., Novak,S., Stockinger,W., Herz,J., Schneider,W.J. and Nimpf,J. (1997) Avian and murine LR8B and human apolipoprotein E receptor 2: differentially spliced products from corresponding genes. Genomics, 42, 185–191. - PubMed
- Brandes C., Kahr,L., Stockinger,W., Hiesberger,T., Schneider,W.J. and Nimpf,J. (2001) Alternative splicing in the ligand binding domain of mouse ApoE receptor-2 produces receptor variants binding reelin but not α2-macroglobulin. J. Biol. Chem., 276, 22160–22169. - PubMed
- Chae T., Kwon,Y.T., Bronson,R., Dikkes,P., Li,E. and Tsai,L.H. (1997) Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures and adult lethality. Neuron, 18, 29–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous