Syndapins integrate N-WASP in receptor-mediated endocytosis - PubMed (original) (raw)
Syndapins integrate N-WASP in receptor-mediated endocytosis
Michael M Kessels et al. EMBO J. 2002.
Abstract
Syndapins are potential links between the cortical actin cytoskeleton and endocytosis because this family of dynamin-associated proteins can also interact with the Arp2/3 complex activator N-WASP. Here we provide evidence for involvement of N-WASP interactions in receptor-mediated endocytosis. We reveal that the observed dominant-negative effects of N-WASP are dependent exclusively on the proline-rich domain, the binding interface of syndapins. Our results therefore suggest that syndapins integrate N-WASP functions in endocytosis. Both proteins co-localize in neuronal cells. Consistent with a crucial role for syndapins in endocytic uptake, co-overexpression of syndapins rescued the endocytosis block caused by N-WASP. An in vivo reconstitution of the syndapin-N-WASP interaction at cellular membranes triggered local actin polymerization. Depletion of endogenous N-WASP by sequestering it to mitochondria or by introducing anti-N-WASP antibodies impaired endocytosis. Our data suggest that syndapins may act as important coordinators of N-WASP and dynamin functions during the different steps of receptor-mediated endocytosis and that local actin polymerization induced by syndapin-N-WASP interactions may be a mechanism supporting clathrin-coated vesicle detachment and movement away from the plasma membrane.
Figures
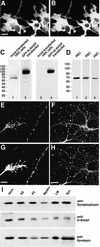
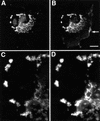
Fig. 1. Syndapins and N-WASP co-localize in growth cones and varicosities of hippocampal neurones. (A and B) Affinity-purified anti-N-WASP antibodies (P337) (A) recognize HA-tagged rat full-length N-WASP expressed in COS-7 cells with the same specificity as monoclonal anti-HA antibodies (B). (C) Lysates from HEK cells either mock-transfected (lanes 1 and 3) or transfected with GFP–N-WASP (lanes 2 and 4) were probed with tag-specific antibodies (anti-GFP, lanes 3 and 4) or affinity-purified P337 antibodies (lanes 1 and 2). (D) Affinity-purified P337 (lane 1) as well as the sera P337 (lane 2) and 3495 (lane 3) recognize a single 70 kDa band in rat brain extracts. (E–H) Syndapin I (G and H) and N-WASP (E and F) co-localize in growth cones (E and G) and varicosities (F and H) in hippocampal neurones cultured for 2 (E and G) and 9 days (F and H), respectively. Scale bars = 20 µm in (A) and (H), and 5 µm in (G). (I) N-WASP and syndapin I are both present in synaptosomal preparations. Western blots of rat brain homogenate (Hom), soluble fraction (S2), crude membrane fraction (P2), myelin fraction (Myelin), light membrane fraction (LM) and synaptosomal fraction (Syn) were probed with antibodies against synaptophysin, N-WASP and syndapin I.
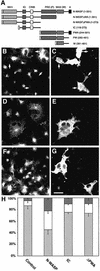
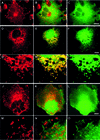
Fig. 2. N-WASP overexpression impairs receptor-mediated endocytosis. (A) Schematic overview of all HA-tagged N-WASP constructs used in the endocytosis assays. (B–G) Images from COS-7 cells transiently transfected with HA-tagged full-length N-WASP (B and C), IC (D and E) and N-WASPΔPWA (F and G), respectively, were recorded by confocal microscopy after an incubation with FITC–transferrin (B, D and F) for 30 min. Expression of N-WASP constructs was visualized with anti-HA antibodies (C, E and G). Arrows in (B) and (C) indicate N-WASP-overexpressing cells with strongly impaired transferrin uptake. Scale bar = 30 µm. (H) Quantitation of the results by assessing the percentages of cells lacking transferrin signal (block; dark grey), displaying significantly reduced levels of uptake (white) and showing endocytosis capabilities similar to untransfected cells (light grey). Untransfected cells, n = 333; N-WASP, n = 401; IC, n = 326; N-WASPΔPWA, n = 334.
Fig. 3. The block of receptor-mediated endocytosis caused by N-WASP overexpression is due to the proline-rich domain. (A–D) Superimposition of FITC–transferrin on staining of the HA-epitope tagged N-WASP fragments. Images were recorded by confocal microscopy from COS-7 cells overexpressing the N-WASP fragments PWA (A), PW (B), W (C) and N-WASPΔWA (D), respectively. Scale bar = 30 µm. (E) Quantitation of the results by assessing the percentages of cells lacking transferrin signal (block, red), displaying significantly reduced levels of uptake (white) and showing endocytosis capabilities similar to untransfected cells (green). Untransfected cells, n = 333; N-WASP, n = 401; PWA, n = 357; PW, n = 399; W, n = 254; N-WASPΔWA, n = 439.
Fig. 4. Syndapin interacts directly with the PRD of N-WASP. (A) Syndapin I and II interactions with N-WASP in the yeast two-hybrid system revealed by the activation of reporter genes assayed via β-gal activity and growth on quadruple drop-out plates (DO). (B) Blot overlay analysis of extracts from mock-transfected and HA-tagged full-length N-WASP-transfected HEK cells with a GST fusion protein of the SH3 domain of syndapin I. (C and D) Extracts from HEK cells transiently transfected with different HA-tagged N-WASP constructs were incubated with a GST–syndapin I SH3 domain-loaded matrix. (C) Immunoblotting of the extracts revealed HA-tagged proteins of the expected sizes. (D) Analysis of proteins bound to matrix-coupled GST–syndapin I SH3 domain. Note that only HA-N-WASP constructs containing the PRD interact with GST–syndapin I SH3. (E) GFP fusion proteins of the PRD and of full-length N-WASP were co-precipitated efficiently by the GST–syndapin I SH3 domain.
Fig. 5. Co-overexpression of syndapin I or II rescues the endo cytosis block caused by overexpression of PRD-containing N-WASP fragments. Quantitation of receptor-mediated endocytosis of FITC– transferrin in COS-7 cells overexpressing full-length syndapins and the PW domains of N-WASP, and co-overexpressing syndapin and PW. Control cells, n = 333; syndapin I, n = 381; PW (N-WASP), n = 399; syndapin I and PW, n = 435; syndapin II-l, n = 341; syndapin II-l and PW, n = 337. A total of 97.4% of the HA-positive cells (stained for the HA-tagged PW domain of N-WASP) were co-expressing Xpress–syndapin I (n = 352) and 76.7% were co-expressing Xpress–syndapin II-l (n = 227), as judged by double immunofluorescence analysis.
Fig. 6. Syndapin fusion proteins encompassing a mitochondrial targeting sequence are recruited efficiently to mitochondrial membranes. COS-7 cells were transfected with mito-syndapin constructs and stained with both MitoTracker® (A and C) and anti-Flag antibodies (B and D). Mito-syndapin is targeted successfully to mitochondria, as best seen in the 3.5× enlargements (C and D) of an area in (A) and (B). A small subpopulation of mito-syndapin fusion proteins can still be observed in actin-rich lamellipodia (arrow in B). Bar = 20 µm.
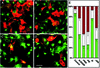
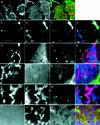
Fig. 7. N-WASP is recruited to mitochondria by mito-syndapin in an SH3 domain-dependent manner. COS-7 cells were transfected with GFP–N-WASP alone (A–C), with GFP–N-WASP plus mito-syndapin (D–I) and with GFP–N-WASP and mito-syndapin(P434L) constructs (J–O), respectively. Both mito-syndapin (D and G) and mito-syndapin(P434L) (J and M) detected with anti-Flag antibodies show a mitochondrial localization pattern. GFP–N-WASP is distributed diffusely and does not co-localize with mitochondria stained by MitoTracker® (A) when transfected alone (see merged image in B) but does localize to mito-syndapin-rich mitochondria (E), as seen well in the 2.7× enlarged details of (D–F) in (G–I). In contrast, in cells co-transfected with mito-syndapin(P434L) (J and M), no such recruitment is observable (K and L), as well seen in the 4× enlarged images (M–O), but GFP–N-WASP shows a rather diffuse localization (L and O). Bars = 10 µm.
Fig. 8. Syndapin binding elicits actin polymerization at sites of intracellular N-WASP recruitment. (A–C) Untransfected cells displaying no F-actin (B) at mitochondria detected by MitoTracker® (A). (D–G) In cells double transfected with mito-syndapin (D) and GFP–N-WASP (E), F-actin (F) can be found at mitochondria (arrows). Cells transfected with mito-syndapin(P434L) and GFP–N-WASP (H–K), with mito-syndapin and GFP–N-WASPΔPRD (L–O), with mito-syndapin and GFP–N-WASPΔWA (P–S) and with a triple combination of mito-syndapin, GFP–N-WASP and HA-WA (T–W) do not show an occurrence of F-actin at mitochondria. F-actin was stained with phalloidin derivatives (B, F, J, N, R and V) and mito-syndapin by anti-Flag antibodies (D, H, L and P). The mito-syndapin staining is omitted in (T–W) and replaced by the anti-HA staining showing the diffuse distribution of HA-WA (T). In the merged images (C, G, K, O, S and W), F-actin is shown in green, GFP–N-WASP in blue and mito-syndapin, MitoTracker® and HA-WA, respectively, in red. Bar in (C) = 5 µm; bar for all other images in (G) = 2.5 µm; n, nucleus.
Fig. 9. Co-overexpression of N-WASP rescues the endocytosis block caused by the syndapin SH3 domain. Quantitation of receptor-mediated endocytosis of FITC–transferrin in COS-7 cells overexpressing the syndapin I SH3 domain alone and in combination with HA-N-WASP compared with control and N-WASP-transfected cells. Control, n = 333; Xpress–syndapin I SH3, n = 379; Xpress–syndapin I SH3 and N-WASP, n = 113; N-WASP, n = 401.
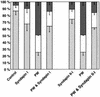
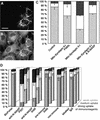
Fig. 10. Depletion of endogenous N-WASP by mitochondrial sequestration or by introduction of anti-N-WASP immunoreagents leads to endocytosis impairments. Expression of mito-syndapin (A) leads to a sequestration of endogenous N-WASP as detected by antibody P337 (B) to mitochondria. (C) Quantitation of receptor-mediated endocytosis of FITC–transferrin in cells overexpressing mito-syndapin II-l(P480L), mito-syndapin II-l, and mito-syndapin II-l and N-WASP. Control, n = 333; mito-syndapin II-l(P480L), n = 325; mito-syndapin II-l, n = 326; mito-syndapin II-l and N-WASP, n = 216. (D) Quantitation of receptor-mediated endocytosis of FITC–transferrin in cells incubated with BioPorter® to introduce immunoreagents. Depicted are the percentages of cells blocked, reduced or wild-type for endocytosis for weak (back row), medium (middle row) and strong uptake (front row) of the respective immunoreagent. Note that the two anti-N-WASP immunoreagents (P337, n = 550; 3495, n = 677) led to dose-dependent impairments of endocytosis, while BioPorter® alone (n = 322) as well as the three control immunoreagents (pre-immune 3495, n = 429; non-immune, n = 555; labelled IgG, n = 670) did not.
Similar articles
- Syndapin isoforms participate in receptor-mediated endocytosis and actin organization.
Qualmann B, Kelly RB. Qualmann B, et al. J Cell Biol. 2000 Mar 6;148(5):1047-62. doi: 10.1083/jcb.148.5.1047. J Cell Biol. 2000. PMID: 10704453 Free PMC article. - The syndapin protein family: linking membrane trafficking with the cytoskeleton.
Kessels MM, Qualmann B. Kessels MM, et al. J Cell Sci. 2004 Jul 1;117(Pt 15):3077-86. doi: 10.1242/jcs.01290. J Cell Sci. 2004. PMID: 15226389 Review. - Impairing actin filament or syndapin functions promotes accumulation of clathrin-coated vesicles at the apical plasma membrane of acinar epithelial cells.
Da Costa SR, Sou E, Xie J, Yarber FA, Okamoto CT, Pidgeon M, Kessels MM, Mircheff AK, Schechter JE, Qualmann B, Hamm-Alvarez SF. Da Costa SR, et al. Mol Biol Cell. 2003 Nov;14(11):4397-413. doi: 10.1091/mbc.e03-05-0315. Epub 2003 Aug 22. Mol Biol Cell. 2003. PMID: 12937279 Free PMC article. - F-BAR proteins of the syndapin family shape the plasma membrane and are crucial for neuromorphogenesis.
Dharmalingam E, Haeckel A, Pinyol R, Schwintzer L, Koch D, Kessels MM, Qualmann B. Dharmalingam E, et al. J Neurosci. 2009 Oct 21;29(42):13315-27. doi: 10.1523/JNEUROSCI.3973-09.2009. J Neurosci. 2009. PMID: 19846719 Free PMC article. - [Reorganization of the actin cytoskeleton by WASP family proteins].
Miki H. Miki H. Seikagaku. 2002 Sep;74(9):1149-61. Seikagaku. 2002. PMID: 12402455 Review. Japanese. No abstract available.
Cited by
- FlnA binding to PACSIN2 F-BAR domain regulates membrane tubulation in megakaryocytes and platelets.
Begonja AJ, Pluthero FG, Suphamungmee W, Giannini S, Christensen H, Leung R, Lo RW, Nakamura F, Lehman W, Plomann M, Hoffmeister KM, Kahr WH, Hartwig JH, Falet H. Begonja AJ, et al. Blood. 2015 Jul 2;126(1):80-8. doi: 10.1182/blood-2014-07-587600. Epub 2015 Apr 2. Blood. 2015. PMID: 25838348 Free PMC article. - Phosphoinositide-binding interface proteins involved in shaping cell membranes.
Takenawa T. Takenawa T. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(5):509-23. doi: 10.2183/pjab.86.509. Proc Jpn Acad Ser B Phys Biol Sci. 2010. PMID: 20467216 Free PMC article. Review. - Syndapin is dispensable for synaptic vesicle endocytosis at the Drosophila larval neuromuscular junction.
Kumar V, Alla SR, Krishnan KS, Ramaswami M. Kumar V, et al. Mol Cell Neurosci. 2009 Feb;40(2):234-41. doi: 10.1016/j.mcn.2008.10.011. Epub 2008 Nov 12. Mol Cell Neurosci. 2009. PMID: 19059483 Free PMC article. - Linkage of the actin cytoskeleton to the postsynaptic density via direct interactions of Abp1 with the ProSAP/Shank family.
Qualmann B, Boeckers TM, Jeromin M, Gundelfinger ED, Kessels MM. Qualmann B, et al. J Neurosci. 2004 Mar 10;24(10):2481-95. doi: 10.1523/JNEUROSCI.5479-03.2004. J Neurosci. 2004. PMID: 15014124 Free PMC article. - RNAi Screen in Tribolium Reveals Involvement of F-BAR Proteins in Myoblast Fusion and Visceral Muscle Morphogenesis in Insects.
Schultheis D, Schwirz J, Frasch M. Schultheis D, et al. G3 (Bethesda). 2019 Apr 9;9(4):1141-1151. doi: 10.1534/g3.118.200996. G3 (Bethesda). 2019. PMID: 30733382 Free PMC article.
References
- Brodin L., Löw,P. and Shupliakov,O. (2000) Sequential steps in clathrin-mediated synaptic vesicle endocytosis. Curr. Opin. Neurobiol., 10, 312–320. - PubMed
- Cremona O. and De Camilli,P. (2001) Phosphoinositides in membrane traffic at the synapse. J. Cell Sci., 114, 1041–1052. - PubMed
- Fujimoto L.M., Roth,R., Heuser,J.E. and Schmid,S.L. (2000) Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic, 1, 161–171. - PubMed
- Higgs H.N. and Pollard,T.D. (1999) Regulation of actin polymerization by Arp2/3 complex and WASp/Scar proteins. J. Biol. Chem., 274, 32531–32534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous