Rab-alphaGDI activity is regulated by a Hsp90 chaperone complex - PubMed (original) (raw)
Rab-alphaGDI activity is regulated by a Hsp90 chaperone complex
Toshiaki Sakisaka et al. EMBO J. 2002.
Abstract
The Rab-specific alphaGDP-dissociation inhibitor (alphaGDI) regulates the recycling of Rab GTPases. We have now identified a novel alphaGDI complex from synaptic membranes that contains three chaperone components: Hsp90, Hsc70 and cysteine string protein (CSP). We find that the alphaGDI-chaperone complex is dissociated in response to Ca(2+)-induced neurotransmitter release, that chaperone complex dissociation is sensitive to the Hsp90 inhibitor geldanamycin (GA) and that GA inhibits the ability of alphaGDI to recycle Rab3A during neurotransmitter release. We propose that alphaGDI interacts with a specialized membrane-associated Rab recycling Hsp90 chaperone system on the vesicle membrane to coordinate the Ca(2+)-dependent events triggering Rab-GTP hydrolysis with retrieval of Rab-GDP to the cytosol.
Figures
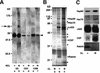
Fig. 1. Identification of an αGDI–Hsp90 chaperone complex. (A) Synaptosomes were incubated in the absence or presence of a non-cleavable cross-linker (NCL). Subsequently, a synaptic soluble (SS) fraction, a crude synaptic vesicle (CSV) fraction and a crude synaptic membrane (CSM) fraction were analyzed for the presence of αGDI by immunoblotting using an anti-αGDI mouse monoclonal antibody. (B) Silver-stained gel of proteins co-immunoprecipitated with αGDI (lanes b and c) or control antibody (a). DPS were treated in the absence or presence of the cleavable cross-linker (CL). Bands denoted by asterisks are unidentified components. (C) Components recovered in anti-αGDI immunoprecipitates (B) were identified by immunoblotting with the indicated antibodies. Results shown are representative of at least three independent experiments.
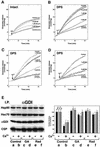
Fig. 2. Dissociation of αGDI from the Hsp90 chaperone complex is dependent on Ca2+-induced neurotransmitter release. (A–C) Requirement for energy and SNARE function for Ca2+-mediated glutamate release from DPS as measured spectrofluorometrically. (A) DPS were incubated in the absence or presence of the ATP-regenerating system (ATP mix), or in the presence of GTPγS or treated with 20 U of apyrase for 15 min at 30°C, washed and assayed for glutamate release in the absence of the ATP mix. (B) Where indicated, synaptosomes were pretreated with NEM before permeabilization. (C) DPS were treated with recombinant light chains of Bot B and E toxins for 15 min at 30°C, and washed prior to Ca2+-stimulated glutamate release. (D) Effects of Bot B and E on dissociation of αGDI from the Hsp90-containing chaperone complex. Untreated (lanes a and b), Bot B-treated (lanes c and d) or Bot E-treated DPS (lanes e and f) were incubated in the absence (a, c and e) or presence (b, d and f) of 10 µM Ca2+ and incubated for 15 min at 30°C. After Ca2+ stimulation, membranes were solubilized and αGDI-containing complexes were immunoprecipitated using the anti-αGDI mouse monoclonal antibody (left panel) and quantitated (right panel) by immunoblotting. Results shown in all panels are representative of at least three independent experiments.
Fig. 3. Dissociation of αGDI from the Hsp90-containing chaperone complex is prevented by GA and radicicol. (A–D) Sensitivity of glutamate release to Hsp90 inhibitors. (A) Intact synaptosomes were pretreated with GA, SB 203580 (SB) or DMSO (as a control) and then depolarized by the addition of KCl (30 mM). (B) Intact synaptosomes were pretreated with GA, SB or DMSO before permeabilization and then stimulated for glutamate release by the addition of Ca2+ (10 µM). (C) Intact synaptosomes were pretreated with GA or DMSO before permeabilization and in the absence or presence of an ATP-regenerating system (ATP mix) and stimulated for glutamate release by the addition of Ca2+. (D) Intact synaptosomes were pretreated with radicicol (Rad) or DMSO before permeabilization and then stimulated for glutamate release by the addition of Ca2+. (E) Effect of GA and radicicol on dissociation of αGDI from the Hsp90-containing chaperone complex. Untreated (lanes a and b), GA-treated (lanes c and d) or radicicol-treated DPS (lanes e and f) were incubated in the absence (a, c and e) or presence (b, d and f) of 10 µM Ca2+ and incubated for 15 min at 30°C. After stimulation, membranes were solubilized and αGDI-containing complexes were immunoprecipitated using the anti-αGDI mouse monoclonal antibody (left panel) and quantitated (right panel) by immunoblotting. The results shown for all panels are representative of at least three independent experiments.
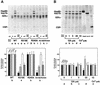
Fig. 4. Formation of αGDI–Hsp90 complexes is ATP dependent and requires the function of the αGDI MEL. (A) Requirement for MEL. Lanes a, d, g and j, His6-αGDI–beads (S) incubated with ATP-regenerating in buffer alone; lanes b, e, h and k, His6-αGDI–beads incubated with purified Hsp90, Hsc70 and recombinant CSP in the presence of the ATP-regenerating system; lanes c, f, i and l, His6-αGDI–beads incubated with purified Hsp90, Hsc70 and CSP in the presence of the ATP-regenerating system containing AMP-PNP instead of ATP. Bound proteins were separated by SDS–PAGE, stained with Coomassie Blue (upper panel) and quantitated (lower panel) by densitometry. (B) Dose-dependent effect of GA on the formation of the Hsp90–αGDI–chaperone complex. His6-αGDI–beads containing wild-type αGDI were incubated in the presence of the ATP-regenerating system with DMSO-treated chaperones (lane a), with the indicated concentration of GA (lanes b–d) treated chaperones (lane e) in the presence of Ca2+. Proteins used for reconstitution are indicated in the left three lanes. Bound proteins were separated by SDS–PAGE, stained with Coomassie Blue (upper panel) and quantitated (lower panel). The results shown in both panels are representative of at least three independent experiments.
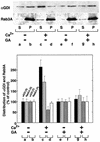
Fig. 5. GA inhibits Ca2+-induced Rab3A recycling. DPS preincubated in the absence (lanes a–d) or presence (lanes e–h) of GA (5 µM) were incubated for 15 min at 30°C in the absence (a, b, e and f) or presence (c, d, g and h) of Ca2+ (10 µM). Following incubation, membranes were collected by centrifugation, and the resulting soluble fraction was immunoprecipitated with anti-αGDI. The immunoprecipitate of the soluble fraction (S) (50% of total shown) and the solubilized membrane pellet fraction (P) (10% of total shown) (upper panel) were quantitated by immunoblotting with anti-αGDI and Rab3A antibodies as indicated (lower panel). The results shown are representative of at least three independent experiments.
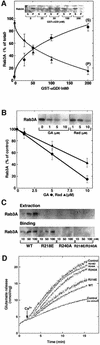
Fig. 6. Rab3A extraction is inhibited by Hsp90 inhibitors and mutation of MEL. (A) Rab3A extraction by GST–αGDI. GST–αGDI at the indicated concentrations was incubated with SM. Membranes were pelleted and the soluble fraction was incubated with GS–beads to recover the released GST–αGDI–Rab3A complex. The recovery of Rab3A associated with either GS–beads (50% of total) (S) (closed circles) or the membrane pellet (50% of total) (P) (closed triangles) was determined by quantitative immunoblotting. (B) Effects of GA and radicicol on Rab3A extraction by GST–αGDI. SM were pretreated with the indicated concentrations of drugs and subsequently incubated with 50 nM GST–αGDI. Recovery quantitated as in (A). (C) Effect of αGDI mutants on Rab3A extraction and binding to Rab3A. Upper panel: SM were incubated with the indicated concentration of the wild type or His6-αGDI mutants. Membranes were pelleted and the supernatant was incubated with cobalt beads to recover the His6-αGDI–Rab3A complex and recovery was determined by quantitative immunoblotting. Lower panel: CHAPS-extracted SM (to release prenylated Rab3A) were incubated with the indicated concentration of wild type or His6-αGDI mutants. Following centrifugation, His6-αGDI was recovered from the supernatant by incubation with cobalt beads and Rab3A binding determined by quantitative immunoblotting. (D) Effects of αGDI mutants on the glutamate release. DPS were preincubated with each of the various αGDI mutants (4 µM final concentration) for 15 min at 4°C, washed and assayed for Ca2+-stimulated glutamate release. The results shown in all panels are representative of at least three independent experiments.
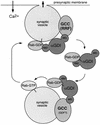
Fig. 7. A working model for αGDI–Hsp90 chaperone interactions in Rab3A recycling. The αGDI–Rab3A cytosolic complex delivers Rab3A to the synaptic vesicle. On the synaptic vesicle, the α
G
DI–Hsp90
c
haperone
c
omplex (GCC) may function as GDF to promote Rab3A dissociation. Following Rab3A release, αGDI is retained on the membrane by GCC through the MEL. During vesicle fusion associated with neurotransmitter release, GCC functions as RRF (Luan et al., 2000). At this step, Rab3A–GDP is extracted from the membrane by sequential steps involving both the RBD of αGDI and the chaperone activity of GCC through MEL. Hsp90 may facilitate the transfer of prenyl groups from the lipid bilayer to αGDI by analogy to steroid hormone receptor pathways.
References
- Alory C. and Balch,W.E. (2001) Organization of the Rab-GD/HM superfamily: the functional basis for choroideremia disease. Traffic, 2, 532–543. - PubMed
- Bittner M.A. and Holz,R.W. (1992) Kinetic analysis of secretion from permeabilized adrenal chromaffin cells reveals distinct components. J. Biol. Chem., 267, 16219–16225. - PubMed
- Blatch G.L. and Lassle,M. (1999) The tetratricopeptide repeat: a structural motif mediating protein–protein interactions. BioEssays, 21, 932–939. - PubMed
- Bronk P., Wenniger,J.J., Dawson-Scully,K., Guo,X., Hong,S., Atwood,H.L. and Zinsmaier,K.E. (2001) Drosophila Hsc70-4 is critical for neurotransmitter exocytosis in vivo. Neuron, 30, 475–488. - PubMed
- Buchner J. (1999) Hsp90 & Co.—a holding for folding. Trends Biochem. Sci., 24, 136–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous