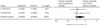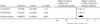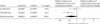The double-edged sword of COX-2 selective NSAIDs - PubMed (original) (raw)
Review
. 2002 Nov 12;167(10):1131-7.
Affiliations
- PMID: 12427705
- PMCID: PMC134294
Review
The double-edged sword of COX-2 selective NSAIDs
James M Wright. CMAJ. 2002.
Abstract
The launch of the cyclooxygenase-2 (COX-2) selective NSAIDs was based on 2 hypotheses: (1) the major adverse effects limiting the usefulness of nonselective NSAIDs are gastrointestinal in nature and (2) COX-2 selective NSAIDs are associated with fewer gastrointestinal adverse effects than nonselective NSAIDs. At the time of the launch, neither of these hypotheses had been proven and, as documented in this review, both remain uncertain. The increased incidence of total and nongastrointestinal serious adverse events, with the COX-2 selective NSAIDs as compared with nonselective NSAIDs, in the Celecoxib Long-term Arthritis Safety Study (CLASS) and the Vioxx Gastrointestinal Outcomes Research (VIGOR) study remains a major concern. The increased morbidity associated with the COX-2 selective NSAIDs may be a manifestation of the COX-2 selectivity of rofecoxib and celecoxib or the supramaximal doses of these drugs used in the trials. Proof that the increased harm was not caused by the COX-2 selectivity of the drugs depends on demonstration in a randomized controlled trial that COX-2 selective NSAIDs at usual doses are as effective as nonselective NSAIDs and cause fewer gastrointestinal serious adverse events without increasing the incidence of total nongastrointestinal serious adverse events.
Figures
Fig. 1: Meta-analysis of relative risk of total mortality with cyclooxygenase-2 (COX-2) selective nonsteroidal anti-inflammatory drugs (NSAIDs) compared with nonselective NSAIDs in the Celecoxib Long-term Arthritis Safety Study (CLASS) , and the Vioxx Gastrointestinal Outcomes Research (VIGOR) study., CI = confidence interval, FDA = US Food and Drug Administration. *Group that received COX-2 selective NSAIDs: number of participants who were affected/total number of participants. †Group that received nonselective NSAIDs: number of participants who were affected/total number of participants.
Fig. 2: Meta-analysis of relative risk of serious adverse events (SAEs) (including death, admission to hospital, and any life- threatening event or event leading to serious disability) with COX-2 selective NSAIDs compared with nonselective NSAIDs in the CLASS , and the VIGOR study.,
Fig. 3: Meta-analysis of relative risk of complicated ulcers with COX-2 selective NSAIDs compared with nonselective NSAIDs in the CLASS , and the VIGOR study.,
Comment in
- Seeking disclosure.
Maksymowych WP. Maksymowych WP. CMAJ. 2003 Apr 15;168(8):960, 962; author reply 962. CMAJ. 2003. PMID: 12695369 Free PMC article. No abstract available. - COX-2 inhibitors and type 4 error.
Pijak MR, Gazdik F. Pijak MR, et al. CMAJ. 2003 Aug 5;169(3):190. CMAJ. 2003. PMID: 12900475 Free PMC article. No abstract available.
Similar articles
- What have we learned from the large outcomes trials of COX-2 selective inhibitors? The rheumatologist's perspective.
Hochberg MC. Hochberg MC. Clin Exp Rheumatol. 2001 Nov-Dec;19(6 Suppl 25):S15-22. Clin Exp Rheumatol. 2001. PMID: 11695246 Review. - Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study.
Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, Geis GS. Silverstein FE, et al. JAMA. 2000 Sep 13;284(10):1247-55. doi: 10.1001/jama.284.10.1247. JAMA. 2000. PMID: 10979111 Clinical Trial. - Are rofecoxib and celecoxib safer NSAIDS?
[No authors listed] [No authors listed] Drug Ther Bull. 2000 Nov;38(11):81-6. Drug Ther Bull. 2000. PMID: 11138599 Review. - A comparison of renal-related adverse drug reactions between rofecoxib and celecoxib, based on the World Health Organization/Uppsala Monitoring Centre safety database.
Zhao SZ, Reynolds MW, Lejkowith J, Whelton A, Arellano FM. Zhao SZ, et al. Clin Ther. 2001 Sep;23(9):1478-91. doi: 10.1016/s0149-2918(01)80121-1. Clin Ther. 2001. PMID: 11589261 - Current perspective on the cardiovascular effects of coxibs.
Konstam MA, Weir MR. Konstam MA, et al. Cleve Clin J Med. 2002;69 Suppl 1:SI47-52. doi: 10.3949/ccjm.69.suppl_1.si47. Cleve Clin J Med. 2002. PMID: 12086293 Review.
Cited by
- Balancing the cyclooxygenase portfolio.
Armstrong PW. Armstrong PW. CMAJ. 2006 May 23;174(11):1581-2. doi: 10.1503/cmaj.060471. CMAJ. 2006. PMID: 16717266 Free PMC article. No abstract available. - Glucosamine therapy for treating osteoarthritis.
Towheed TE, Maxwell L, Anastassiades TP, Shea B, Houpt J, Robinson V, Hochberg MC, Wells G. Towheed TE, et al. Cochrane Database Syst Rev. 2005 Apr 18;2005(2):CD002946. doi: 10.1002/14651858.CD002946.pub2. Cochrane Database Syst Rev. 2005. PMID: 15846645 Free PMC article. Review. - Cardiovascular events associated with long-term use of celecoxib, rofecoxib and meloxicam in Taiwan: an observational study.
Huang WF, Hsiao FY, Tsai YW, Wen YW, Shih YT. Huang WF, et al. Drug Saf. 2006;29(3):261-72. doi: 10.2165/00002018-200629030-00009. Drug Saf. 2006. PMID: 16524325 - COX-2 inhibitors and type 4 error.
Pijak MR, Gazdik F. Pijak MR, et al. CMAJ. 2003 Aug 5;169(3):190. CMAJ. 2003. PMID: 12900475 Free PMC article. No abstract available. - Effect of Hydrotalcite on Indometacin-Induced Gastric Injury in Rats.
Fang YF, Xu WL, Wang L, Lian QW, Qiu LF, Zhou H, Chen SJ. Fang YF, et al. Biomed Res Int. 2019 Apr 11;2019:4605748. doi: 10.1155/2019/4605748. eCollection 2019. Biomed Res Int. 2019. PMID: 31111054 Free PMC article.
References
- Lipsky PE. Progress toward a new class of therapeutics: selective COX-2 inhibition. J Rheumatol 1997;24(Suppl 49):1-8. - PubMed
- Lane NE. Pain management in osteoarthritis: the role of COX-2 inhibitors. J Rheumatol 1997;24(Suppl 49):20-4. - PubMed
- Celecoxib (Celebrex): Is it a breakthrough drug? Ther Lett 1999;31:1-2. Available: www.ti.ubc.ca/pages/letter31.htm (accessed 2002 Sept 12)
- Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. JAMA 2000;284:1247-55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials