Rab11a and myosin Vb regulate recycling of the M4 muscarinic acetylcholine receptor - PubMed (original) (raw)
Rab11a and myosin Vb regulate recycling of the M4 muscarinic acetylcholine receptor
Laura A Volpicelli et al. J Neurosci. 2002.
Abstract
Agonist-induced internalization followed by subsequent return to the cell surface regulates G-protein-coupled receptor (GPCR) activity. Because the cellular responsiveness to ligand depends on the balance between receptor degradation and recycling, it is crucial to identify the molecules involved in GPCR recovery to the cell surface. In this study, we identify mechanisms involved in the recycling of the M4 subtype of muscarinic acetylcholine receptor. M4 is highly expressed in the CNS, plays a role in locomotor activity, and is a novel therapeutic target for neurologic and psychiatric disorders. Previous studies show that, after cholinergic stimulation, M4 internalizes from the cell surface to endosomes in cell culture and the rat brain. Here, we show that, after activation, M4 traffics to transferrin receptor- and Rab11a-positive perinuclear endosomes. Expression of the constitutively GDP-bound, inactive mutant Rab11aS25N inhibits M4 trafficking to recycling endosomes. Expression of the C-terminal tail of myosin Vb, a Rab11a effector, enhances M4 accumulation in perinuclear endosomes. Both Rab11aS25N and the myosin Vb tail impair M4 recycling. The results demonstrate that GPCR recycling is mediated through a discrete pathway using both Rab11a and myosin Vb.
Figures
Fig. 1.
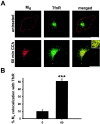
After agonist stimulation, M4 traffics to a perinuclear compartment, in which it colocalizes with the TfnR.A, In untreated PC12 cells, M4(red) localized to the cell surface, and the TfnR (green) showed a primarily perinuclear distribution. After 60 min continuous CCh treatment, M4redistributed from the cell surface to the perinuclear compartment, in which it colocalized extensively with the TfnR (visualized as_yellow_ in the merged images). The _inset_shows a higher-magnification image of the perinuclear compartment, demonstrating that the majority of internalized M4colocalized with the TfnR. Scale bar, 10 μm. B, Quantitation of confocal images demonstrated that M4 showed minimal colocalization with TfnR in untreated cells (n = 11), and, after 60 min CCh treatment, M4 overlap with TfnR was significantly enhanced (n = 16). ***p < 0.001.
Fig. 2.
M4 returns to the cell surface after agonist stimulation and washout. To selectively examine the amount of M4 that recycles to the cell surface after CCh treatment and washout, cells were treated with cycloheximide to prevent new receptor synthesis, and cycloheximide was included in all subsequent drug treatments. A, Cells were untreated, treated continuously with CCh for 60 min, or treated with CCh for 60 min, rinsed, and incubated in media alone for 60 min to allow M4 recovery to the cell surface. In untreated cells, M4 (red) localized primarily to the cell surface, but a small proportion of receptors also showed an intracellular localization. Cell surface M4 colocalized (yellow in the merged image) with the plasma membrane marker Na+/K+ ATPase (green). The inset shows a higher-magnification image of the cell surface, demonstrating that, although M4 showed colocalization with the Na+/K+ ATPase (yellow), subdomains exist at the cell surface that contain M4 but not the Na+/K+ ATPase (red). Although all cells show staining for the Na+/K+ ATPase, not all PC12 cells show M4 staining, demonstrating heterogeneity of M4 receptor expression in PC12 cells. After 60 min continual CCh treatment, M4 trafficked from the cell surface to perinuclear endosomes and no longer colocalized with Na+/K+ ATPase. After 60 min CCh treatment followed by 60 min washout, M4 returned to the cell surface, in which it colocalized with the Na+/K+ ATPase. A proportion of receptors also showed an intracellular distribution. Scale bars, 10 μm. B, Measurements of M4 recycling using quantitative immunocytochemistry were compared with measurements of mAChR recycling using 3H-NMS. Cells were treated with CCh, rinsed, and incubated in media alone for 60 or 90 min. The amounts of cell surface receptors measured using both assays after CCh washout were normalized to the amounts of cell surface receptors in untreated cells (black bars and cross-hatched bars). When expressed as a percentage of untreated cells, the extent of M4 recycling measured by quantitative immunocytochemistry was slightly higher than the extent of recycling measured by binding assays. Subtracting the residual M4remaining after 60 min CCh treatment, however, produced values for M4 recycling using confocal images (gray bars) that were similar to measurements of mAChR recycling using binding assays. This method of calculating M4recycling in confocal images was thus used throughout the remainder of the paper. C, The amount of M4 recovery to the cell was quantified using confocal images at various time points after CCh washout. The graph demonstrates that M4 began to return to the cell surface as early as 15 min after agonist washout, and, 3 hr after washout, the majority of M4 (visible by immunocytochemistry) localized to the cell surface.
Fig. 3.
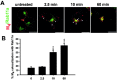
After prolonged CCh stimulation, M4colocalizes with Rab11a in the perinuclear compartment.A, After 2.5 min continual CCh treatment, M4(red) began to redistribute from the cell surface to large puncta (arrowheads) that did not colocalize with Rab11a (green). M4 began to colocalize with Rab11a after 10 min CCh treatment (visualized as_yellow_, arrow), and, after 60 min, M4 colocalized extensively with Rab11a in the perinuclear compartment. Scale bars, 10 μm. B, Quantitation of confocal images demonstrated that M4 showed minimal colocalization with Rab11a in untreated cells (n = 7) and after 2.5 min CCh stimulation (n = 4). By 10 min of continual CCh treatment (n = 9), M4 overlap with Rab11a was significantly enhanced and increased further after 60 min CCh treatment (n = 12). ***p < 0.001.
Fig. 4.
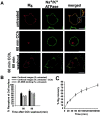
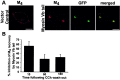
Constitutively GDP-bound Rab11aS25N prevents M4 accumulation in perinuclear recycling endosomes. Cells were treated continuously with CCh for 60 min. A, In control cells transfected with the pEGFP vector, GFP showed a diffuse, ubiquitous distribution, and M4 (red) localized to the perinuclear compartment. In cells transfected with GFP-Rab11aS25N, GFP showed a diffuse, cytosolic distribution consistent with the inability of GDP-bound Rab11aS25N to bind membrane. In contrast to vector-transfected control cells, M4 in cells expressing GFP-Rab11aS25N localized to small puncta distributed throughout the cell and did not accumulate in the perinuclear compartment. Scale bars, 10 μm. B, In pEGFP vector-transfected control cells, M4 (red) and TfnR (green) localized to the perinuclear compartment. Expression of GFP-Rab11aS25N caused dispersal of both M4 and TfnR to small puncta distributed throughout the cell. M4 and TfnR showed colocalization in both vector-transfected control cells and GFP-Rab11aS25N-expressing cells. Scale bars, 10 μm.
Fig. 5.
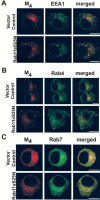
Constitutively GDP-bound Rab11aS25N does not prevent M4 transit through early endosomes or enhance M4 trafficking to late endosomes. Cells were treated continuously with CCh for 60 min. A, In vector-transfected control cells, the early endosomal marker EEA1 (green) localized to puncta distributed peripherally throughout the cell, and M4 and EEA1 showed minimal colocalization. In cells transfected with GFP-Rab11aS25N, M4 localized to small puncta dispersed throughout the cell. However, M4 and EEA1 showed little colocalization. Scale bars, 10 μm. B, Rab4 (green) localized to small puncta peripherally distributed throughout the cell. M4 (red) showed minimal colocalization with Rab4 in vector-transfected control cells and cells expressing Rab11aS25N. Scale bars, 10 μm. C, The late endosomal marker Rab7 localized to small puncta distributed throughout the cells. In vector-transfected control cells, internalized M4 showed some colocalization with Rab7. In cells expressing GFP-Rab11aS25N, M4 colocalization with Rab7 was not enhanced. Scale bars, 10 μm.
Fig. 6.
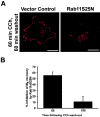
Constitutively GDP-bound Rab11aS25N impairs M4 recycling. Cells were treated with CCh for 60 min, rinsed, and incubated in media alone for 60 or 180 min.A, After CCh treatment and washout, in cells expressing Rab11aS25N, M4 showed little recovery to the cell surface compared with vector-transfected control cells and localized to small, intracellular puncta. Scale bars, 10 μm. B, The amount of M4 recovery to the cell surface was measured by quantitation of confocal images, and the percentage of inhibition of M4 recycling by Rab11aS25N was calculated relative to vector-transfected control cells. M4 recycling was dramatically inhibited by Rab11aS25N expression 60 min after agonist washout. However, M4 eventually returned to the cell surface 3 hr after agonist washout such that the percentage of inhibition of M4 recycling was minimal after this prolonged time point.
Fig. 7.
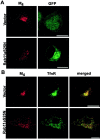
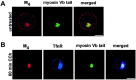
The myosin Vb tail enhances M4accumulation in perinuclear endosomes. A, Myosin Vb tail-GFP showed a concentrated, perinuclear localization. In untreated cells expressing the myosin Vb tail, M4(red) localized to the cell surface but also showed some accumulation intracellularly. Scale bar, 10 μm. B, Expression of the myosin Vb tail enhanced M4 concentration in the perinuclear compartment after 60 min CCh treatment. The TfnR (blue) also showed enhanced accumulation in the perinuclear compartment, and M4, the TfnR, and the myosin Vb tail (green) colocalized extensively (visualized as white in the merged image). Scale bar, 10 μm.
Fig. 8.
The myosin Vb tail prevents M4recycling. A, Cells were treated with CCh for 60 min, rinsed, and incubated in media alone for 60 min. As expected, in vector-transfected control cells, M4 returned to the cell surface. In cells expressing the myosin Vb tail, the majority of M4 remained in perinuclear recycling endosomes, although a small amount of M4 returned to the cell surface. Scale bars, 10 μm. B, The amount of M4 recovery to the cell surface was measured by quantitation of confocal images, and the percentage of inhibition of M4 recycling by the myosin Vb tail was calculated relative to vector-transfected control cells. Expression of the myosin Vb tail dramatically inhibited M4 recycling 15 min after CCh washout. M4recycling remained impaired by the myosin Vb tail for 60 min and as long as 180 min after CCh washout.
Similar articles
- Rab5-dependent trafficking of the m4 muscarinic acetylcholine receptor to the plasma membrane, early endosomes, and multivesicular bodies.
Volpicelli LA, Lah JJ, Levey AI. Volpicelli LA, et al. J Biol Chem. 2001 Dec 14;276(50):47590-8. doi: 10.1074/jbc.M106535200. Epub 2001 Oct 4. J Biol Chem. 2001. PMID: 11590149 - Myosin vb is associated with plasma membrane recycling systems.
Lapierre LA, Kumar R, Hales CM, Navarre J, Bhartur SG, Burnette JO, Provance DW Jr, Mercer JA, Bähler M, Goldenring JR. Lapierre LA, et al. Mol Biol Cell. 2001 Jun;12(6):1843-57. doi: 10.1091/mbc.12.6.1843. Mol Biol Cell. 2001. PMID: 11408590 Free PMC article. - Myosin Vb is required for trafficking of the cystic fibrosis transmembrane conductance regulator in Rab11a-specific apical recycling endosomes in polarized human airway epithelial cells.
Swiatecka-Urban A, Talebian L, Kanno E, Moreau-Marquis S, Coutermarsh B, Hansen K, Karlson KH, Barnaby R, Cheney RE, Langford GM, Fukuda M, Stanton BA. Swiatecka-Urban A, et al. J Biol Chem. 2007 Aug 10;282(32):23725-36. doi: 10.1074/jbc.M608531200. Epub 2007 Apr 26. J Biol Chem. 2007. PMID: 17462998 - Rab11-family interacting protein 2 and myosin Vb are required for CXCR2 recycling and receptor-mediated chemotaxis.
Fan GH, Lapierre LA, Goldenring JR, Sai J, Richmond A. Fan GH, et al. Mol Biol Cell. 2004 May;15(5):2456-69. doi: 10.1091/mbc.e03-09-0706. Epub 2004 Mar 5. Mol Biol Cell. 2004. PMID: 15004234 Free PMC article. - A Rab10-ACAP1-Arf6 GTPases cascade modulates M4 muscarinic acetylcholine receptor trafficking and signaling.
Xu R, Wan M, Shi X, Ma S, Zhang L, Yi P, Zhang R. Xu R, et al. Cell Mol Life Sci. 2023 Mar 14;80(4):87. doi: 10.1007/s00018-023-04722-x. Cell Mol Life Sci. 2023. PMID: 36917255 Free PMC article.
Cited by
- Regulation of apical constriction via microtubule- and Rab11-dependent apical transport during tissue invagination.
Le TP, Chung S. Le TP, et al. Mol Biol Cell. 2021 May 1;32(10):1033-1047. doi: 10.1091/mbc.E21-01-0021. Epub 2021 Mar 31. Mol Biol Cell. 2021. PMID: 33788621 Free PMC article. - Fast modulation of μ-opioid receptor (MOR) recycling is mediated by receptor agonists.
Roman-Vendrell C, Yu YJ, Yudowski GA. Roman-Vendrell C, et al. J Biol Chem. 2012 Apr 27;287(18):14782-91. doi: 10.1074/jbc.M111.319616. Epub 2012 Feb 29. J Biol Chem. 2012. PMID: 22378794 Free PMC article. - Coaggregation of RNA-binding proteins in a model of TDP-43 proteinopathy with selective RGG motif methylation and a role for RRM1 ubiquitination.
Dammer EB, Fallini C, Gozal YM, Duong DM, Rossoll W, Xu P, Lah JJ, Levey AI, Peng J, Bassell GJ, Seyfried NT. Dammer EB, et al. PLoS One. 2012;7(6):e38658. doi: 10.1371/journal.pone.0038658. Epub 2012 Jun 21. PLoS One. 2012. PMID: 22761693 Free PMC article. - Rab11 GTPase-regulated membrane trafficking is crucial for tip-focused pollen tube growth in tobacco.
de Graaf BH, Cheung AY, Andreyeva T, Levasseur K, Kieliszewski M, Wu HM. de Graaf BH, et al. Plant Cell. 2005 Sep;17(9):2564-79. doi: 10.1105/tpc.105.033183. Epub 2005 Aug 12. Plant Cell. 2005. PMID: 16100336 Free PMC article. - Regulation of G protein-coupled receptor export trafficking.
Dong C, Filipeanu CM, Duvernay MT, Wu G. Dong C, et al. Biochim Biophys Acta. 2007 Apr;1768(4):853-70. doi: 10.1016/j.bbamem.2006.09.008. Epub 2006 Sep 23. Biochim Biophys Acta. 2007. PMID: 17074298 Free PMC article. Review.
References
- Anborgh PH, Seachrist JL, Dale LB, Ferguson SS. Receptor/beta-arrestin complex formation and the differential trafficking and resensitization of beta2-adrenergic and angiotensin II type 1A receptors. Mol Endocrinol. 2000;14:2040–2053. - PubMed
- Berkeley JL, Levey AI. Muscarinic activation of mitogen-activated protein kinase in PC12 cells. J Neurochem. 2000;75:487–493. - PubMed
- Bernard V, Levey AI, Bloch B. Regulation of the subcellular distribution of m4 muscarinic acetylcholine receptors in striatal neurons in vivo by the cholinergic environment: evidence for regulation of cell surface receptors by endogenous and exogenous stimulation. J Neurosci. 1999;19:10237–10249. - PMC - PubMed
- Bloch B, Dumartin B, Bernard V. In vivo regulation of intraneuronal trafficking of G protein-coupled receptors for neurotransmitters. Trends Pharmacol Sci. 1999;20:315–319. - PubMed
- Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, Shannon HE, Tollefson GD, Rasmussen K, Bymaster FP, Hurley DJ, Potter WZ, Paul SM. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54:465–473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK048370/DK/NIDDK NIH HHS/United States
- DK43405/DK/NIDDK NIH HHS/United States
- R01 DK043405/DK/NIDDK NIH HHS/United States
- R01 NS030454/NS/NINDS NIH HHS/United States
- R01 NS30454/NS/NINDS NIH HHS/United States
- Z01 DK043405/ImNIH/Intramural NIH HHS/United States
- DK48370/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources