Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice - PubMed (original) (raw)
Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice
Orly Lazarov et al. J Neurosci. 2002.
Abstract
A neuropathological hallmark of Alzheimer's disease is the deposition of amyloid-beta (Abeta) peptides in senile plaques in the hippocampus and cerebral cortex. Abeta is derived from larger integral membrane proteins termed amyloid precursor proteins (APP). We demonstrated previously that APP, synthesized by neurons in the entorhinal cortex, is transported via the perforant pathway to presynaptic terminals in the dentate gyrus. We reported that, although full-length APP and membrane-tethered, C-terminal APP derivatives (APP-CTFs) accumulate at terminal fields, the production of Abeta peptides at these sites was indeterminate. To test the hypothesis that APP-CTFs, generated from axonally transported APP, are further metabolized to Abeta peptides that are subsequently released and deposited proximal to nerve terminals, we created unilateral knife lesions of the perforant pathway of transgenic mice that exhibit hippocampal amyloid deposits. We observed pronounced reductions in amyloid burden in the ipsilateral dentate gyrus, findings that lead us to conclude that axonally transported APP gives rise to Abeta peptides that are released from presynaptic sites in the dentate gyrus and deposited in extracellular plaques. Moreover, our findings are consistent with the view that Abeta deposits are dynamic structures and that the perforant path lesion alters the equilibrium between Abeta production-deposition toward clearance as a consequence of blocked axonal transport of APP from the entorhinal cortex to terminal fields in the hippocampus.
Figures
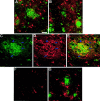
Fig. 1.
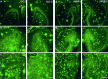
Representative images of amyloid immunoreactivity in the dentate gyrus (left panels; DG) and hippocampus (right panels; HIPP) of unlesioned transgenic mice (A, 6-month-old mouse;B, 8-month-old mouse; C, 13-month-old mouse). Amyloid immunoreactivity in the left side (DG-I,HIPP-I) was compared with that detected in the contralateral (right) side (DG-C,HIPP-C). Amyloid was detected by immunolabeling with 6E10 antibodies. No significant difference in amyloid immunoreactivity could be detected. Scale bars, 250 μm.
Fig. 2.
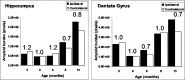
Quantitative analysis of amyloid burden in the hippocampus of unlesioned mice. For each individual animal analyzed, amyloid burden in the left side was compared with the burden in the right side in both the hippocampus (left) and the dentate gyrus (right). The ratio between the hemispheres is indicated. No difference in amyloid burden could be observed.
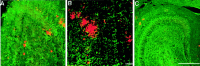
Fig. 3.
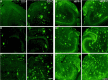
Representative images of amyloid immunoreactivity in the dentate gyrus (left panels; DG) and hippocampus (right panel; HIPP) of perforant pathway-lesioned mice (A, 7-month-old mouse;B, C, 10-month-old mice). Amyloid immunoreactivity in the ipsilateral (lesioned) side (DG-I, HIPP-I) was compared with that in the contralateral side (DG-C,HIPP-C) 1 month after perforant pathway lesion. Amyloid was detected by immunolabeling with 6E10 antibodies. Immunoreactivity of amyloid deposits is reduced in the ipsilateral hippocampus and dentate gyrus compared with the contralateral side. Scale bars, 250 μm.
Fig. 4.
Quantitative analysis of amyloid burden in the hippocampus of perforant pathway-lesioned mice 1 month after lesion. Analysis revealed a significant reduction in amyloid burden in the ipsilateral hippocampus (left) and dentate gyrus (right) compared with the contralateral side (the ratio between the hemispheres is indicated). The reduction in amyloid burden in the dentate gyrus was most pronounced.
Fig. 5.
Amyloid burden in the ipsilateral and contralateral hippocampus and dentate gyrus of stab wound-treated mice 1 month after treatment. Top panels show 6E10-labeled hippocampus of a stab wound-treated mouse. _Bottom panels_shows quantitative analysis of amyloid burden in the ipsilateral (HIPP-I) and contralateral (HIPP-C) hippocampus and dentate gyrus of stab wound-treated mice 1 month after treatment. No significant difference in amyloid burden was found between the ipsilateral and the contralateral hippocampus or dentate gyrus in these animals. Scale bar, 250 μm.
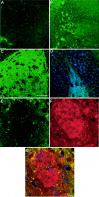
Fig. 6.
A, B, Reactive gliosis in the hippocampus and dentate gyrus of unlesioned mice as could be detected by immunostaining using S100β antibodies in dentate gyrus in the left hemisphere (A) and in the right one (B). S100β immunoreactivity (in_red_) was most pronounced around amyloid deposits (immunostained using 6E10 antibodies; in green). Some overlap between S100β-labeled glia (D) and amyloid deposits (6E10 antibodies; C) could be detected (E, see arrows). F,G, Reactive morphology of glia is reduced in the ipsilateral hippocampus (F) but not in the contralateral one (G) 1 month after perforant pathway lesion. Scale bar, 150 μm.
Fig. 7.
Neurofilament morphology in the dentate gyrus–hippocampus as detected by immunolabeling 1 month after perforant pathway lesion. A, B, Contralateral hippocampus (B, high power).C, Ipsilateral hippocampus. Brain sections are double immunolabeled for neurofilament (in green) and β-amyloid (6E10 antibodies; in red). Neuritic atrophy could be detected in the contralateral side. In contrast, neurofilament morphology in the ipsilateral side seems to be recovered, at least in part. Scale bars, 250 μm.
Fig. 8.
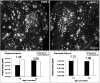
Quantitative analysis of amyloid burden in the hippocampus of mice after reinnervation of the outer molecular layer of the dentate gyrus. Mice were subject to perforant pathway lesion at 4 months of age. Four months later, brain sections were analyzed for amyloid burden. The ratio between the hemispheres is indicated. No difference in amyloid burden could be observed between the lesioned hippocampus and the unlesioned one.
Fig. 9.
Reactive synaptogenesis in the outer molecular layer of mice 4 months after perforant pathway lesion. Brain sections were examined for the presence of the presynaptic marker Bassoon 1 week (A), 1 month (B), or 4 months (C, D) after lesion. Immunoreactivity for Bassoon was easily detected in the ipsilateral hippocampus of 4 month postlesion mice, less pronounced in brain sections of 1 month mice, and barely detected in sections of 1 week postlesioned mice. D, Bassoon and synapsin double-labeled synapses in the dentate gyrus of 4 month postlesion mice. E–G, Synapses could be detected, surrounding amyloid deposits. This close proximity may suggest that the source of deposited material is synapse released. E, Bassoon immunoreactivity in the dentate gyrus of 4 month postlesion mice.F, Amyloid immunoreactivity at the same area as_E_, as detected by FCA3542 antibodies. G, Merged image of Bassoon and amyloid immunoreactivities.Arrows in G indicate overlapping staining of immunoreactivity for Aβ42 and Bassoon. Scale bars, 250 μm.
Similar articles
- Disruption of corticocortical connections ameliorates amyloid burden in terminal fields in a transgenic model of Abeta amyloidosis.
Sheng JG, Price DL, Koliatsos VE. Sheng JG, et al. J Neurosci. 2002 Nov 15;22(22):9794-9. doi: 10.1523/JNEUROSCI.22-22-09794.2002. J Neurosci. 2002. PMID: 12427835 Free PMC article. - Alzheimer amyloid protein precursor in the rat hippocampus: transport and processing through the perforant path.
Buxbaum JD, Thinakaran G, Koliatsos V, O'Callahan J, Slunt HH, Price DL, Sisodia SS. Buxbaum JD, et al. J Neurosci. 1998 Dec 1;18(23):9629-37. doi: 10.1523/JNEUROSCI.18-23-09629.1998. J Neurosci. 1998. PMID: 9822724 Free PMC article. - Diffuse amyloid deposition, but not plaque number, is reduced in amyloid precursor protein/presenilin 1 double-transgenic mice by pathway lesions.
van Groen T, Liu L, Ikonen S, Kadish I. van Groen T, et al. Neuroscience. 2003;119(4):1185-97. doi: 10.1016/s0306-4522(03)00215-x. Neuroscience. 2003. PMID: 12831872 - APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis.
Crews L, Rockenstein E, Masliah E. Crews L, et al. Brain Struct Funct. 2010 Mar;214(2-3):111-26. doi: 10.1007/s00429-009-0232-6. Epub 2009 Nov 29. Brain Struct Funct. 2010. PMID: 20091183 Free PMC article. Review. - [The lesions of Alzheimer's disease: which therapeutic perspectives?].
Duyckaerts C, Perruchini C, Lebouvier T, Potier MC. Duyckaerts C, et al. Bull Acad Natl Med. 2008 Feb;192(2):303-18; discussion 318-21. Bull Acad Natl Med. 2008. PMID: 18819685 Review. French.
Cited by
- An APP ectodomain mutation outside of the Aβ domain promotes Aβ production in vitro and deposition in vivo.
Zhang X, Zhang CM, Prokopenko D, Liang Y, Zhen SY, Weigle IQ, Han W, Aryal M, Tanzi RE, Sisodia SS. Zhang X, et al. J Exp Med. 2021 Jun 7;218(6):e20210313. doi: 10.1084/jem.20210313. J Exp Med. 2021. PMID: 33822840 Free PMC article. - Alzheimer precursor protein interaction with the Nogo-66 receptor reduces amyloid-beta plaque deposition.
Park JH, Gimbel DA, GrandPre T, Lee JK, Kim JE, Li W, Lee DH, Strittmatter SM. Park JH, et al. J Neurosci. 2006 Feb 1;26(5):1386-95. doi: 10.1523/JNEUROSCI.3291-05.2006. J Neurosci. 2006. PMID: 16452662 Free PMC article. - DHA diet reduces AD pathology in young APPswe/PS1 Delta E9 transgenic mice: possible gender effects.
Perez SE, Berg BM, Moore KA, He B, Counts SE, Fritz JJ, Hu YS, Lazarov O, Lah JJ, Mufson EJ. Perez SE, et al. J Neurosci Res. 2010 Apr;88(5):1026-40. doi: 10.1002/jnr.22266. J Neurosci Res. 2010. PMID: 19859965 Free PMC article. - Cholinergic forebrain degeneration in the APPswe/PS1DeltaE9 transgenic mouse.
Perez SE, Dar S, Ikonomovic MD, DeKosky ST, Mufson EJ. Perez SE, et al. Neurobiol Dis. 2007 Oct;28(1):3-15. doi: 10.1016/j.nbd.2007.06.015. Epub 2007 Jun 27. Neurobiol Dis. 2007. PMID: 17662610 Free PMC article. - The Role of NMDA Receptors in Alzheimer's Disease.
Liu J, Chang L, Song Y, Li H, Wu Y. Liu J, et al. Front Neurosci. 2019 Feb 8;13:43. doi: 10.3389/fnins.2019.00043. eCollection 2019. Front Neurosci. 2019. PMID: 30800052 Free PMC article. Review.
References
- Adami C, Sorci G, Blasi E, Agneletti AL, Bistoni F, Donato R. S100B expression in and effects on microglia. Glia. 2001;33:131–142. - PubMed
- Amaratunga A, Fine RE. Generation of amyloidogenic C-terminal fragments during rapid axonal transport in vivo of beta-amyloid precursor protein in the optic nerve. J Biol Chem. 1995;270:17268–17272. - PubMed
- Barelli H, Lebeau A, Vizzavona J, Delaere P, Chevallier N, Drouot C, Marambaud P, Ancolio K, Baxbaun JD, Khorkova O, Heroux J, Sahasrabudhe S, Martinez J, Warter JM, Mohr M, Checler F. Characterization of new polyclonal antibodies specific for 40 and 42 amino acid-long amyloid beta peptides: their age to examine the cell biology of presenting and the immunohistochemistry of sporadic Alzheimer's disease and cerebral amyloid angiopathy cases. Mol Med. 1997;3:695–707. - PMC - PubMed
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. - PubMed
- Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases