The presenilins - PubMed (original) (raw)
Review
The presenilins
Anurag Tandon et al. Genome Biol. 2002.
Abstract
The presenilins are evolutionarily conserved transmembrane proteins that regulate cleavage of certain other proteins in their transmembrane domains. The clinical significance of this regulation is shown by the contribution of presenilin mutations to 20-50% of early-onset cases of inherited Alzheimer's disease. Although the precise molecular mechanism underlying presenilin function or dysfunction remains elusive, presenilins are thought to be part of a complex of proteins that has 'gamma-secretase cleavage' activity, which is clearly central in the pathogenesis of Alzheimer's disease. Mutations in presenilins increase the production of the longer isoforms of amyloid beta peptide, which are neurotoxic and prone to self-aggregation. Biochemical studies indicate that the presenilins do not act alone but operate within large heteromeric protein complexes, whose components and enzymatic core are the subject of much study and controversy; one essential component is nicastrin. The presenilin primary sequence is remarkably well conserved in eukaryotes, suggesting some functional conservation; indeed, defects caused by mutations in the nemotode presenilin homolog can be rescued by human presenilin.
Figures
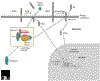
Figure 1
A molecular model of Presenilin-1. The protein is thought to have eight transmembrane domains. Residues associated with mutations found in familial Alzheimer's disease are colored as indicated in the key. 'Endoproteolysis' indicates the approximate site of the imprecise cleavage of the molecule.
Figure 2
The role of presenilins in the γ-secretase cleavage of Notch and βAPP. Notch is cleaved by tumor necrosis factor α converting enzyme (TACE), and its ligand binds to the part of Notch that remains attached to the membrane. βAPP is cleaved by either the γ-secretase pathway or the γ-secretase pathway to give a membrane-bound carboxy-terminal fragment (APP-CTF). Subsequent γ-secretase cleavage (in the transmembrane domain) of Notch or APP-CTF produces carboxy-terminal intracellular domains, NICD and AICD, respectively, which enter the nucleus and are thought to regulate gene expression. The γ-secretase cleavage of βAPP also produces the neurotoxic Aβ peptide, but only if βAPP has been first cleaved by γ-secretase (not γ-secretase). The γ-secretase complex includes, in addition to PS1, the presenilin-binding protein nicastrin; members of the Armadillo protein family, such as β-catenin, have also been detected in presenilin complexes, although their role is not understood. Aph-1 and Pen-2 may also participate in the γ-secretase complex.
Similar articles
- gamma-Secretase inhibitors as molecular probes of presenilin function.
Wolfe MS. Wolfe MS. J Mol Neurosci. 2001 Oct;17(2):199-204. doi: 10.1385/JMN:17:2:199. J Mol Neurosci. 2001. PMID: 11816793 Review. - Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity.
Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Wolfe MS, et al. Nature. 1999 Apr 8;398(6727):513-7. doi: 10.1038/19077. Nature. 1999. PMID: 10206644 - Secretase as a target for Alzheimer's disease.
Wolfe MS. Wolfe MS. Curr Top Med Chem. 2002 Apr;2(4):371-83. doi: 10.2174/1568026024607535. Curr Top Med Chem. 2002. PMID: 11966461 Review. - Presenilins as therapeutic targets for the treatment of Alzheimer's disease.
Golde TE, Younkin SG. Golde TE, et al. Trends Mol Med. 2001 Jun;7(6):264-9. doi: 10.1016/s1471-4914(01)02064-0. Trends Mol Med. 2001. PMID: 11378516 Review. - A portrait of Alzheimer secretases--new features and familiar faces.
Esler WP, Wolfe MS. Esler WP, et al. Science. 2001 Aug 24;293(5534):1449-54. doi: 10.1126/science.1064638. Science. 2001. PMID: 11520976 Review.
Cited by
- Rutin Potentially Binds the Gamma Secretase Catalytic Site, Down Regulates the Notch Signaling Pathway and Reduces Sphere Formation in Colonospheres.
Singh AK, Shuaib M, Prajapati KS, Kumar S. Singh AK, et al. Metabolites. 2022 Sep 29;12(10):926. doi: 10.3390/metabo12100926. Metabolites. 2022. PMID: 36295828 Free PMC article. - Probable novel PSEN2 Val214Leu mutation in Alzheimer's disease supported by structural prediction.
Youn YC, Bagyinszky E, Kim H, Choi BO, An SS, Kim S. Youn YC, et al. BMC Neurol. 2014 May 15;14:105. doi: 10.1186/1471-2377-14-105. BMC Neurol. 2014. PMID: 24885952 Free PMC article. - Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer's disease-linked presenilin mutants in human cells and mouse neurons.
Cheung KH, Mei L, Mak DO, Hayashi I, Iwatsubo T, Kang DE, Foskett JK. Cheung KH, et al. Sci Signal. 2010 Mar 23;3(114):ra22. doi: 10.1126/scisignal.2000818. Sci Signal. 2010. PMID: 20332427 Free PMC article. - Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1.
Nelson O, Tu H, Lei T, Bentahir M, de Strooper B, Bezprozvanny I. Nelson O, et al. J Clin Invest. 2007 May;117(5):1230-9. doi: 10.1172/JCI30447. Epub 2007 Apr 12. J Clin Invest. 2007. PMID: 17431506 Free PMC article. - Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations.
Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Tu H, et al. Cell. 2006 Sep 8;126(5):981-93. doi: 10.1016/j.cell.2006.06.059. Cell. 2006. PMID: 16959576 Free PMC article.
References
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K. Cloning of a gene bearing mis-sense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–760. This study revealed the involvement of presenilin-1 in early onset FAD. - PubMed
- Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature. 1995;376:775–778. The authors identify mutations in the PS2 gene as a pathogenic factor in AD. - PubMed
- Levy-Lahad E, Wijsman EM, Nemens E, Anderson L, Goddard KA, Weber JL, Bird TD, Schellenberg GD. A familial Alzheimer's disease locus on chromosome 1. Science. 1995;269:970–973. Mapping of a familial AD locus to the PS2 gene. - PubMed
- The Alzheimer's disease collaborative group. The structure of the presenilin 1 (S182) gene and the identification of six novel mutations in early onset AD pedigrees. Nat Genet. 1995;11:219–222. The first analysis of the intron-exon structure of the PS1 gene. - PubMed
- Hutton M, Busfield F, Wragg M, Crook R, Perez-tur J, Clark RF, Prihar G, Talbot C, Phillips H, Wright K, et al. Complete analysis of the presenilin 1 gene in early onset Alzheimer's disease. Neuroreport. 1996;7:801–805. An analysis of the intron-exon structure of the PS1 gene. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources