Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system - PubMed (original) (raw)
Comparative Study
. 2002 Nov 26;99(24):15620-5.
doi: 10.1073/pnas.232565399. Epub 2002 Nov 12.
Affiliations
- PMID: 12429857
- PMCID: PMC137766
- DOI: 10.1073/pnas.232565399
Comparative Study
Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system
Jonathan Kipnis et al. Proc Natl Acad Sci U S A. 2002.
Abstract
The ability of rats or mice to withstand the consequences of injury to myelinated axons in the CNS was previously shown to depend on the ability to manifest a T cell-mediated protective immune response, which is amenable to boosting by myelin-specific T cells. Here we show that this ability, assessed by retinal ganglion cell survival after optic nerve injury or locomotor activity after spinal cord contusion, is decreased if the animals were immunized as neonates with myelin proteins (resulting in their nonresponsiveness as adults to myelin proteins) or injected with naturally occurring regulatory CD4(+)CD25(+) T cells immediately after the injury, and is improved by elimination of these regulatory T cells. In nude BALBc mice replenished with a splenocyte population lacking CD4(+)CD25(+) regulatory T cells, significantly more neurons survived after optic nerve injury than in nude mice replenished with a complete splenocyte population or in matched wild-type controls. In contrast, neuronal survival in wild-type BALBc mice injected with CD4(+)CD25(+) regulatory T cells immediately after injury was significantly worse than in noninjected controls. These findings suggest that the ability to cope with the sequelae of a CNS insult is affected unfavorably by nonresponsiveness to myelin self-antigens and favorably by conditions allowing rapid expression of an autoimmune response. The regulatory T cells might represent an evolutionary compromise between the need to avoid autoimmune diseases and the need for autoimmunity on alert for the purpose of tissue maintenance.
Figures
Fig 1.
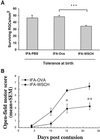
Neonatal tolerance to myelin antigens abolishes spontaneous neuroprotection after optic nerve crush injury and spinal cord contusion. One-day-old SPD rats were tolerized to myelin antigens by i.p. injection, with 400 μg of WSCH emulsified in IFA. As adults, the rats were subjected to two kinds of CNS injury: (A) Partial crush injury of the optic nerve: the numbers of surviving neurons were significantly lower in the rats tolerized to myelin antigens than in control rats that were tolerized 1 day after birth with ovalbumin (OVA) or were not tolerized (P < 0.01 and P < 0.001, respectively). The figure shows the results of a representative experiment, one of two independent experiments (in each experiment n = 6–8 rats in each group). (B) Contusive injury of the spinal cord at the level of T9. Recovery was assessed by the locomotor activity score. The locomotor scores of myelin-tolerized contused rats were significantly lower than those of OVA-tolerized rats (n = 5 rats in each group; P < 0.01; two-tailed Student's t test).
Fig 2.
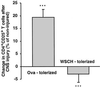
Nonresponsiveness to CNS injury in rats neonatally immunized with spinal cord homogenate. Three days after being subjected to CNS injury the spleens were removed from rats and verified for T cell activation by using CD25 as an activation marker (ergotope)-α-chain of IL-2 receptor. CNS injury triggered activation of T cells in naïve rats and rats immunized at birth with nonmyelin proteins (Ova-tolerized), whereas activation of T cells was not observed in rats neonatally immunized with myelin proteins (WSCH-tolerized). The results are the mean of three experiments (n = 3 in each group in each experiment; P < 0.001).
Fig 3.
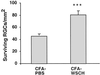
Immunization of rats with WSCH improves neuronal survival after optic nerve crush injury. Four days before optic nerve injury, Lewis rats were immunized in the hind footpads with 3 mg of WSCH emulsified in CFA supplemented with 5 mg/ml Mycobacterium tuberculosis H37Ra. Retrograde labeling of the RGCs showed that significantly more neurons survived in rats immunized with WSCH than in PBS-injected rats. The results shown are of one representative experiment of three independent experiments (in each experiment, n = 7–8 in each group; P < 0.001, two-tailed Student's t test).
Fig 4.
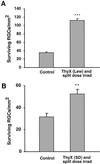
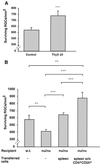
Thymectomy followed by split-dose irradiation in rats improves neuronal survival. Lewis (A) and SPD (B) rats (4 weeks old) were thymectomized (ThyX) and then subjected to split-dose irradiation (four bursts of 250 rad each at 2-week intervals). Immediately after the last irradiation the rats were subjected to partial optic nerve crush injury. Neuronal survival was determined 2 weeks later by application of a fluorescent dye. Significantly more neurons survived in the irradiated thymectomized Lewis rats (A) than in rats subjected to injury only. The results shown are of one representative experiment of three independent experiments (in each experiment, n = 7 in each group; P < 0.001, two-tailed Student's t test). (B) Significantly more neurons survived in the irradiated thymectomized SPD rats than in SPD rats subjected to injury only (n = 9 in each group; P < 0.01, two-tailed Student's t test).
Fig 5.
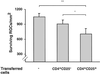
Depletion of naturally occurring regulatory CD4+CD25+ T cells in BALB/c mice improves neuronal survival after optic nerve crush injury. (A) BALB/c mice were thymectomized (ThyX) 3 days after birth to deplete their regulatory T cells and were subjected as adults to severe unilateral crush injury inflicted on the intraorbital portion of the optic nerve. Surviving neurons were labeled by the application, 3 days before injury, of the neurotracer dye FluoroGold. Significantly more neurons survived in the thymectomized mice than in control (nonthymectomized) age-matched mice (n = 7–8 in each group; P < 0.001; Student's t test). (B) Neuronal survival after optic nerve crush injury in BALB/c nu/nu mice (devoid of T cells) was worse than in wild-type mice of matched background. Endogenous neuroprotection in the nude mice was restored by injection of 5 × 107 wild-type splenocytes. Injection of splenocytes depleted of regulatory CD4+CD25+ T cells increased neuronal survival in these mice beyond even that seen in the wild type (n = 5–6 in each group; P values between different groups, obtained by two-tailed Student's t test, are indicated by asterisks above the graph bars; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
Fig 6.
Naturally occurring regulatory CD4+CD25+ T cells diminish spontaneous neuroprotection_._ BALB/c mice, endowed with the spontaneous ability to evoke a protective beneficial autoimmune response, were injected with 2 × 106 purified activated regulatory CD4+CD25+ T cells. Control mice were injected with 2 × 106 purified activated effector cells (CD4+CD25−) or with PBS. Injection of regulatory T cells had an adverse effect on neuronal survival (more neurons underwent secondary degeneration). The results shown are of one representative experiment of three independent experiments; n = 5–6 mice in each group (P < 0.01 and 0.05; respectively).
Similar articles
- Bacterial DNA confers neuroprotection after optic nerve injury by suppressing CD4+CD25+ regulatory T-cell activity.
Johnson TV, Camras CB, Kipnis J. Johnson TV, et al. Invest Ophthalmol Vis Sci. 2007 Aug;48(8):3441-9. doi: 10.1167/iovs.06-1351. Invest Ophthalmol Vis Sci. 2007. PMID: 17652711 - Low-dose gamma-irradiation promotes survival of injured neurons in the central nervous system via homeostasis-driven proliferation of T cells.
Kipnis J, Avidan H, Markovich Y, Mizrahi T, Hauben E, Prigozhina TB, Slavin S, Schwartz M. Kipnis J, et al. Eur J Neurosci. 2004 Mar;19(5):1191-8. doi: 10.1111/j.1460-9568.2004.03207.x. Eur J Neurosci. 2004. PMID: 15016077 - Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells.
Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, Schwartz M, Kipnis J. Cohen H, et al. J Neurobiol. 2006 May;66(6):552-63. doi: 10.1002/neu.20249. J Neurobiol. 2006. PMID: 16555237 - Regulatory T cells generated ex vivo as an approach for the therapy of autoimmune disease.
Horwitz DA, Zheng SG, Gray JD, Wang JH, Ohtsuka K, Yamagiwa S. Horwitz DA, et al. Semin Immunol. 2004 Apr;16(2):135-43. doi: 10.1016/j.smim.2003.12.009. Semin Immunol. 2004. PMID: 15036237 Review. - Immune regulation by CD4+CD25+ regulatory T cells: implications for transplantation tolerance.
Taams L, Vukmanovic-Stejic M, Salmon M, Akbar A. Taams L, et al. Transpl Immunol. 2003 Jul-Sep;11(3-4):277-85. doi: 10.1016/S0966-3274(03)00047-9. Transpl Immunol. 2003. PMID: 12967781 Review.
Cited by
- Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer's disease pathology.
Baruch K, Rosenzweig N, Kertser A, Deczkowska A, Sharif AM, Spinrad A, Tsitsou-Kampeli A, Sarel A, Cahalon L, Schwartz M. Baruch K, et al. Nat Commun. 2015 Aug 18;6:7967. doi: 10.1038/ncomms8967. Nat Commun. 2015. PMID: 26284939 Free PMC article. - Central Nervous System-Peripheral Immune System Dialogue in Neurological Disorders: Possible Application of Neuroimmunology in Urology.
Park HS, Park MJ, Kwon MS. Park HS, et al. Int Neurourol J. 2016 May;20(Suppl 1):S8-14. doi: 10.5213/inj.1632614.307. Epub 2016 May 26. Int Neurourol J. 2016. PMID: 27230462 Free PMC article. Review. - Immunosuppression and Neuroinflammation in Stroke Pathobiology.
Jiang Q, Stone CR, Elkin K, Geng X, Ding Y. Jiang Q, et al. Exp Neurobiol. 2021 Apr 30;30(2):101-112. doi: 10.5607/en20033. Exp Neurobiol. 2021. PMID: 33972464 Free PMC article. Review. - T-cell-based vaccination for morphological and functional neuroprotection in a rat model of chronically elevated intraocular pressure.
Bakalash S, Ben-Shlomo G, Aloni E, Shaked I, Wheeler L, Ofri R, Schwartz M. Bakalash S, et al. J Mol Med (Berl). 2005 Nov;83(11):904-16. doi: 10.1007/s00109-005-0689-6. Epub 2005 Aug 12. J Mol Med (Berl). 2005. PMID: 16096740 - Inflammation and immunomodulation in central nervous system injury - B cells as a novel therapeutic opportunity.
Maheshwari S, Dwyer LJ, Sîrbulescu RF. Maheshwari S, et al. Neurobiol Dis. 2023 May;180:106077. doi: 10.1016/j.nbd.2023.106077. Epub 2023 Mar 11. Neurobiol Dis. 2023. PMID: 36914074 Free PMC article. Review.
References
- Schwartz M. & Kipnis, J. (2001) Trends Mol. Med. 7 252-258. - PubMed
- Butovsky O., Hauben, E. & Schwartz, M. (2001) FASEB J. 15 1065-1067. - PubMed
- Moalem G., Leibowitz-Amit, R., Yoles, E., Mor, F., Cohen, I. R. & Schwartz, M. (1999) Nat. Med. 5 49-55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials