Clustering of neuronal potassium channels is independent of their interaction with PSD-95 - PubMed (original) (raw)
Clustering of neuronal potassium channels is independent of their interaction with PSD-95
Matthew N Rasband et al. J Cell Biol. 2002.
Abstract
Voltage-dependent potassium channels regulate membrane excitability and cell-cell communication in the mammalian nervous system, and are found highly localized at distinct neuronal subcellular sites. Kv1 (mammalian Shaker family) potassium channels and the neurexin Caspr2, both of which contain COOH-terminal PDZ domain binding peptide motifs, are found colocalized at high density at juxtaparanodes flanking nodes of Ranvier of myelinated axons. The PDZ domain-containing protein PSD-95, which clusters Kv1 potassium channels in heterologous cells, has been proposed to play a major role in potassium channel clustering in mammalian neurons. Here, we show that PSD-95 colocalizes precisely with Kv1 potassium channels and Caspr2 at juxtaparanodes, and that a macromolecular complex of Kv1 channels and PSD-95 can be immunopurified from mammalian brain and spinal cord. Surprisingly, we find that the high density clustering of Kv1 channels and Caspr2 at juxtaparanodes is normal in a mutant mouse lacking juxtaparanodal PSD-95, and that the indirect interaction between Kv1 channels and Caspr2 is maintained in these mutant mice. These data suggest that the primary function of PSD-95 at juxtaparanodes lies outside of its accepted role in mediating the high density clustering of Kv1 potassium channels at these sites.
Figures
Figure 1.
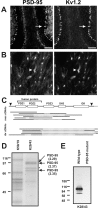
PSD-95 is present at juxtaparanodes and colocalizes with Kv1.2. (A and B) Immunoreactivity for PSD-95 (K28/43 mAb) and Kv1.2 colocalize at cerebellar basket cell terminals (A, arrows) and juxtaparanodes (B, arrowheads). (C) Schematic showing the full-length cDNA for dlg4 (PSD-95), including translation start and end sites (arrowheads), and the region corresponding to the fusion protein used to generate the K28 mAbs. Expression cloning from mouse spinal cord (msc) and rat brain (rbr) libraries resulted in the depicted cDNAs, all of which corresponded to PSD-95. Sequenced regions for each clone are shown as solid lines. (D) Purification of PSD-95 using a K28/43 immunoaffinity column. The major proteins purified on the K28/43 column were compared with an unrelated IgG precolumn (K59/19), excised, digested with trypsin, and the masses of the resulting peptides were determined by mass spectrometry. Search of the protein databases using ProFound yielded high probability matches (Z values given in parentheses) to PSD-95 for three of the four high molecular mass bands. The unidentified band (asterisk) did not significantly match any protein in the databases. (E) mAb K28/43 immunoblot of brain homogenate from wild-type and mutant PSD-95 mice; 20 μg of protein were loaded in each lane. PDZ, PSD-95, discs-large, zona occludens; SH3, Src homology 3; GK, guanylate kinase. Bars: (A) 100 μm, (B) 10 μm.
Figure 2.
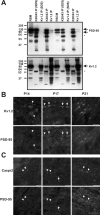
Kv1.2 and PSD-95 form a macromolecular protein complex in vivo. (A) Immunoprecipitation reactions performed on RBM and RSCM, using a pAb against Kv1.2 and an mAb against PSD-95 (K28/43). Immunoblotting for PSD-95 (top) and Kv1.2 (bottom) show that they are coimmunoprecipitated, and that this interaction is sensitive to the presence of SDS in the extraction buffer. The mouse IgG band is indicated by an asterisk. 50 μg of crude membranes was loaded in the RBM and RSCM lanes, and immunoprecipitated proteins from the equivalent of 100 μg of membranes were loaded for each IP lane. (B and C) Developmental coexpression of Kv1.2, PSD-95, and Caspr2 at rat optic nerve juxtaparanodes using indirect immunofluorescence. Kv1.2 (B) and Caspr2 (C) were detected as early as P14 (arrowheads), but PSD-95 was not seen to colocalize with this staining until P17 and later at P21. At P17, both more mature (arrows) and immature (arrowheads) juxtaparanodes were detected.
Figure 3.
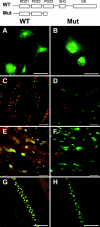
Kv1.2 is clustered in PSD-95 mutant animals. (A and B) K28/43 immunostaining of COS-1 fibroblasts expressing wild-type PSD-95 (WT; A) or the truncated form of PSD-95 (Mut; B), corresponding to that present in the PSD-95 mutant mouse (cartoon at top of this figure). (C and D) Immunolabeling of WT (C) and mutant (D) mouse optic nerves with mAb K28/43 against PSD-95 (red) and Nav1.6 (green). (E and F) PSD-95 (red) and Kv1.2 (green) colocalize in optic nerves from WT mice, but in mutant mice only clustered Kv1.2 is detected (green). (G and H) At basket cell terminals, PSD-95 (red) and Kv1.2 (green) colocalize in WT mice, but in mutant mice only clustered Kv1.2 is detected (green). Bars: (A and B) 50 μm; (C–F) 10 μm; (G and H) 100 μm. PDZ, PSD-95, discs-large, zona occludens; SH3, Src homology 3; GK, guanylate kinase.
Figure 4.
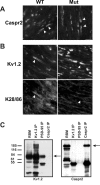
Immunostaining of WT and PSD-95 mutant mice, and immunoprecipitation (IP) of Kv1.2, PSD-95, and Caspr2 from RBM. (A) Caspr2 is clustered in both WT and mutant mice (arrowheads). (B) Although Kv1.2 is clustered in both WT and PSD-95 mutant mice (B_,_ top), the pan-MAGUK antibody K28/86 only stains juxtaparanodes in WT mice (arrowheads). (C) Coimmunoprecipitation from RBM using pAbs against Kv1.2 and Caspr2, and mAb K28/43 that recognizes PSD-95. Arrows indicate the positions of the respective antigens. The RBM lanes were loaded with 50 μg of crude membrane protein, and each immunoprecipitation lane was loaded with precipitated proteins from 500 μg of starting material. Bars, 10 μm.
Figure 5.
Immunoprecipitation reactions and immunoblotting from wild-type and PSD-95 mutant mouse brain membranes show that Kv1 channels and Caspr2 interact independently of PSD-95. (A) Coimmunoprecipitation reactions in Triton X-100 or SML detergent containing buffers. WT and Mut brain lanes were loaded with 50 μg of crude brain membranes (1-min exposure shown), and each immunoprecipitation lane was loaded with precipitated proteins from 250 μg of starting brain membranes (1-h exposure shown). Immunoblots were probed with monoclonal anti-Kv1.2 or anti-Caspr2 rabbit serum. (B) Detergent-insoluble membrane pellets from WT and Mut mice obtained after extraction in buffers containing the indicated concentrations of Triton X-100 (% vol/vol) were assayed by immunoblots probed with antibodies against Kv1.2 or PSD-95. Each lane represents the detergent-insoluble material from 100 μg of starting brain membrane.
Figure 6.
Model of the molecular components of the juxtaparanode. The juxtaparanodal protein complex includes the known components PSD-95, Caspr2, and Kv1 channels (α and β subunits). Additional components likely include a putative glial binding partner for Caspr2 (possibly TAG-1, a PDZ-domain containing scaffolding protein linking Caspr2 and Kv1 channels) and cytoskeletal proteins. The paranodal axoglial junctions are also shown because these structures are essential for the localization of juxtaparanodal proteins.
Similar articles
- Multiple molecular interactions determine the clustering of Caspr2 and Kv1 channels in myelinated axons.
Horresh I, Poliak S, Grant S, Bredt D, Rasband MN, Peles E. Horresh I, et al. J Neurosci. 2008 Dec 24;28(52):14213-22. doi: 10.1523/JNEUROSCI.3398-08.2008. J Neurosci. 2008. PMID: 19109503 Free PMC article. - ADAM22, a Kv1 channel-interacting protein, recruits membrane-associated guanylate kinases to juxtaparanodes of myelinated axons.
Ogawa Y, Oses-Prieto J, Kim MY, Horresh I, Peles E, Burlingame AL, Trimmer JS, Meijer D, Rasband MN. Ogawa Y, et al. J Neurosci. 2010 Jan 20;30(3):1038-48. doi: 10.1523/JNEUROSCI.4661-09.2010. J Neurosci. 2010. PMID: 20089912 Free PMC article. - Differential K+ channel clustering activity of PSD-95 and SAP97, two related membrane-associated putative guanylate kinases.
Kim E, Sheng M. Kim E, et al. Neuropharmacology. 1996;35(7):993-1000. doi: 10.1016/0028-3908(96)00093-7. Neuropharmacology. 1996. PMID: 8938729 - It's "juxta" potassium channel!
Rasband MN. Rasband MN. J Neurosci Res. 2004 Jun 15;76(6):749-57. doi: 10.1002/jnr.20073. J Neurosci Res. 2004. PMID: 15160387 Review. - Assembly and Function of the Juxtaparanodal Kv1 Complex in Health and Disease.
Pinatel D, Faivre-Sarrailh C. Pinatel D, et al. Life (Basel). 2020 Dec 24;11(1):8. doi: 10.3390/life11010008. Life (Basel). 2020. PMID: 33374190 Free PMC article. Review.
Cited by
- Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers.
Traka M, Goutebroze L, Denisenko N, Bessa M, Nifli A, Havaki S, Iwakura Y, Fukamauchi F, Watanabe K, Soliven B, Girault JA, Karagogeos D. Traka M, et al. J Cell Biol. 2003 Sep 15;162(6):1161-72. doi: 10.1083/jcb.200305078. J Cell Biol. 2003. PMID: 12975355 Free PMC article. - Developing a Toolbox of Antibodies Validated for Array Tomography-Based Imaging of Brain Synapses.
Micheva KD, Gong B, Collman F, Weinberg RJ, Smith SJ, Trimmer JS, Murray KD. Micheva KD, et al. eNeuro. 2023 Dec 22;10(12):ENEURO.0290-23.2023. doi: 10.1523/ENEURO.0290-23.2023. Print 2023 Dec. eNeuro. 2023. PMID: 37945352 Free PMC article. - Benefits and pitfalls of secondary antibodies: why choosing the right secondary is of primary importance.
Manning CF, Bundros AM, Trimmer JS. Manning CF, et al. PLoS One. 2012;7(6):e38313. doi: 10.1371/journal.pone.0038313. Epub 2012 Jun 1. PLoS One. 2012. PMID: 22675541 Free PMC article. - Composition, assembly, and maintenance of excitable membrane domains in myelinated axons.
Rasband MN. Rasband MN. Semin Cell Dev Biol. 2011 Apr;22(2):178-84. doi: 10.1016/j.semcdb.2010.09.010. Epub 2010 Oct 12. Semin Cell Dev Biol. 2011. PMID: 20932927 Free PMC article. Review. - Voltage-gated ion channels in the axon initial segment of human cortical pyramidal cells and their relationship with chandelier cells.
Inda MC, DeFelipe J, Muñoz A. Inda MC, et al. Proc Natl Acad Sci U S A. 2006 Feb 21;103(8):2920-5. doi: 10.1073/pnas.0511197103. Epub 2006 Feb 10. Proc Natl Acad Sci U S A. 2006. PMID: 16473933 Free PMC article.
References
- Arroyo, E.J., and S.S. Scherer. 2000. On the molecular architecture of myelinated fibers. Histochem. Cell Biol. 113:1–18. - PubMed
- Arroyo, E.J., T. Xu, S. Poliak, M. Watson, E. Peles, and S.S. Scherer. 2001. Internodal specializations of myelinated axons in the central nervous system. Cell Tissue Res. 305:53–66. - PubMed
- Baba, H., H. Akita, T. Ishibashi, Y. Inoue, K. Nakahira, and K. Ikenaka. 1999. Completion of myelin compaction, but not the attachment of oligodendroglial processes triggers K+ channel clustering. J. Neurosci. Res. 58:752–764. - PubMed
- Bekele-Arcuri, Z., M.F. Matos, L. Manganas, B.W. Strassle, M.M. Monaghan, K.J. Rhodes, and J.S. Trimmer. 1996. Generation and characterization of subtype-specific monoclonal antibodies to K+ channel α- and β-subunit polypeptides. Neuropharmacology. 35:851–865. - PubMed
- Berghs, S., D. Aggujaro, R. Dirkx, E. Maksimova, P. Stabach, J.M. Hermel, J.P. Zhang, W. Philbrick, V. Slepnev, T. Ort, and M. Solimena. 2000. βIV spectrin, a new spectrin localized at axon initial segments and nodes of Ranvier in the central and peripheral nervous system. J. Cell Biol. 151:985–1002. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous