Cross-linking the B7 family molecule B7-DC directly activates immune functions of dendritic cells - PubMed (original) (raw)
Cross-linking the B7 family molecule B7-DC directly activates immune functions of dendritic cells
Loc T Nguyen et al. J Exp Med. 2002.
Retraction in
- Cross-linking the B7 family molecule B7-DC directly activates immune functions of dendritic cells.
Nguyen LT, Radhakrishnan S, Ciric B, Tamada K, Shin T, Pardoll DM, Chen L, Rodriguez M, Pease LR. Nguyen LT, et al. J Exp Med. 2010 Apr 12;207(4):901. doi: 10.1084/jem.2002146632610r. Epub 2010 Apr 5. J Exp Med. 2010. PMID: 20368577 Free PMC article. No abstract available.
Abstract
B7-DC molecules are known to function as ligands on antigen-presenting cells (APCs), enhancing T cell activation. In this study, cross-linking B7-DC with the monoclonal antibody sHIgM12 directly potentiates dendritic cell (DC) function by enhancing DC presentation of major histocompatibility complex-peptide complexes, promoting DC survival; and increasing secretion of interleukin (IL)-12p70, a key T helper cell type 1 promoting cytokine. Furthermore, ex vivo treatment of DCs or systemic treatment of mice with sHIgM12 increases the number of transplanted DCs that reach draining lymph nodes and increases the ability of lymph node APCs to activate naive T cells. Systemic administration of the antibody has an equivalent effect on DCs transferred at a distant site. These findings implicate B7-DC expressed on DCs in bidirectional communication. In addition to the established costimulatory and inhibitory functions associated with B7-DC, this molecule can also function as a conduit for extracellular signals to DCs modifying DC functions.
Figures
Figure 1.
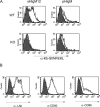
B7-DC cross-linking increases antigen presentation in vitro, but does not increase expression of cell surface accessory molecules. (A) Presentation of Kb-SIINFEKL complexes following treatment of ovalbumin protein-pulsed DCs with sHIgM12 (left panels) or pHIgM (right panel) antibodies. Bone marrow–derived DCs from wild-type (top panels) or B7-DC−/− (bottom panels) mice were stained with 25-D1.16 antibody (unshaded) to detect specific MHC–peptide complexes. Shaded histogram represents unpulsed DCs. (B) Immunostaining of ovalbumin-pulsed DCs for MHC class II, CD80, or CD86 molecules after sHIgM12 (solid line) and pHIgM (hashed line) antibody treatment. Shaded histogram represents staining isotype control antibody.
Figure 2.
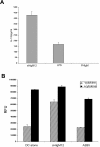
Murine bone marrow–derived DCs secrete high levels of IL-12p70 and exhibit prolonged survival in vitro following B7-DC cross-linking. (A) Day 7 bone marrow–derived DCs were treated with sHIgM12, polyclonal IgM control antibody or LPS at a final concentration of 10 μg/ml. The supernatants were collected 72 h after stimulation and an ELISA (BD Biosciences) was performed for the active fraction of IL-12 cytokine. The experimental groups were tested in triplicates and at numerous dilutions with each demonstrating a similar profile. (B) DC metabolism after treatment was accessed by reduction of Alamar Blue after a 24 h incubation period. DCs were plated on day 5 into 96-well plates at 2 × 104 cells/well. Cells were cultured with sHIgM12, A2B5 control antibody, or media with (solid) or without IL-4/GM-CSF (hashed) supplementation. The data is representative of three separate plates with each data point done in triplicate.
Figure 3.
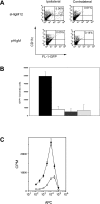
Anti-B7.DC treatment enhances potency and number of adoptively-transferred DCs recovered from draining lymph node. (A) Flow cytometric analysis, and (B) enumeration of CD11c+; GFP+ cells in draining lymph nodes of treated mice. Treatment with sHIgM12 (solid); or pIgM (hashed). Isolation of cells from ipsilateral (dark); or contralateral (light) lymph nodes. (C) Proliferation of OT-I TCR Tg splenocytes after incubation with isolated DCs. Ovalbumin-pulsed DCs were treated before adoptive transfer with either sHIgM12 (filled) or pIgM (open) control. Each group was performed in triplicate. The data is representative of three independent experiments.
Figure 4.
Systemic sHIgM12 treatment increases the number of adoptively transferred DCs recovered from the draining lymph node. Untreated GFP+ DCs were injected (day 0) into the footpad of mice that had received intravenous injections of sHIgM12 or pHIgM on days –1, 0, +1. Ipsilateral and contralateral draining lymph nodes were recovered 48 h later. Staining and flow cytometric analysis was performed as described in Materials and Methods.
Similar articles
- Naturally occurring human IgM antibody that binds B7-DC and potentiates T cell stimulation by dendritic cells.
Radhakrishnan S, Nguyen LT, Ciric B, Ure DR, Zhou B, Tamada K, Dong H, Tseng SY, Shin T, Pardoll DM, Chen L, Kyle RA, Rodriguez M, Pease LR. Radhakrishnan S, et al. J Immunol. 2003 Feb 15;170(4):1830-8. doi: 10.4049/jimmunol.170.4.1830. J Immunol. 2003. PMID: 12574348 Retracted. - B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells.
Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. Tseng SY, et al. J Exp Med. 2001 Apr 2;193(7):839-46. doi: 10.1084/jem.193.7.839. J Exp Med. 2001. PMID: 11283156 Free PMC article. - Dendritic cells activated by cross-linking B7-DC (PD-L2) block inflammatory airway disease.
Radhakrishnan S, Iijima K, Kobayashi T, Kita H, Pease LR. Radhakrishnan S, et al. J Allergy Clin Immunol. 2005 Sep;116(3):668-74. doi: 10.1016/j.jaci.2005.04.038. J Allergy Clin Immunol. 2005. PMID: 16159641 Retracted. - Cytokines in the generation and maturation of dendritic cells: recent advances.
Zou GM, Tam YK. Zou GM, et al. Eur Cytokine Netw. 2002 Apr-Jun;13(2):186-99. Eur Cytokine Netw. 2002. PMID: 12101074 Review. - The Actin Cytoskeleton at the Immunological Synapse of Dendritic Cells.
Rodríguez-Fernández JL, Criado-García O. Rodríguez-Fernández JL, et al. Front Cell Dev Biol. 2021 Aug 2;9:679500. doi: 10.3389/fcell.2021.679500. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34409027 Free PMC article. Review.
Cited by
- Co-expression of PD-L1 and p-AKT is associated with poor prognosis in diffuse large B-cell lymphoma via PD-1/PD-L1 axis activating intracellular AKT/mTOR pathway in tumor cells.
Dong L, Lv H, Li W, Song Z, Li L, Zhou S, Qiu L, Qian Z, Liu X, Feng L, Meng B, Fu K, Wang X, Pan-Hammarström Q, Wang P, Wang X, Zhang H. Dong L, et al. Oncotarget. 2016 May 31;7(22):33350-62. doi: 10.18632/oncotarget.9061. Oncotarget. 2016. PMID: 27147575 Free PMC article. - Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells.
Gao Y, Nish SA, Jiang R, Hou L, Licona-Limón P, Weinstein JS, Zhao H, Medzhitov R. Gao Y, et al. Immunity. 2013 Oct 17;39(4):722-32. doi: 10.1016/j.immuni.2013.08.028. Epub 2013 Sep 26. Immunity. 2013. PMID: 24076050 Free PMC article. - Immunotherapy of cancer in 2012.
Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Kirkwood JM, et al. CA Cancer J Clin. 2012 Sep-Oct;62(5):309-35. doi: 10.3322/caac.20132. Epub 2012 May 10. CA Cancer J Clin. 2012. PMID: 22576456 Free PMC article. Review. - Dendritic cells in cancer immunotherapy: vaccines or autologous transplants?
Kalinski P, Edington H, Zeh HJ, Okada H, Butterfield LH, Kirkwood JM, Bartlett DL. Kalinski P, et al. Immunol Res. 2011 Aug;50(2-3):235-47. doi: 10.1007/s12026-011-8224-z. Immunol Res. 2011. PMID: 21717071 Free PMC article. - Co-inhibitory molecules: Controlling the effectors or controlling the controllers?
Thangavelu G, Smolarchuk C, Anderson CC. Thangavelu G, et al. Self Nonself. 2010 Apr;1(2):77-88. doi: 10.4161/self.1.2.11548. Epub 2010 Feb 16. Self Nonself. 2010. PMID: 21487510 Free PMC article.
References
- Coyle, A.J., and J.C. Gutierrez-Ramos. 2001. The expanding B7 superfamily: increasing complexity in costimulatory signals regulating T cell function. Nat. Immunol. 2:203–209. - PubMed
- Henry, J., M.M. Miller, and P. Pontarotti. 1999. Structure and evolution of the extended B7 family. Immunol. Today. 20:285–288. - PubMed
- Dong, H., G. Zhou, K. Tamada, and L. Chen. 1999. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 5:1365–1369. - PubMed
- Latchman, Y., C.R. Wood, T. Chernova, D. Chaudhary, M. Borde, I. Chernova, Y. Iwai, A.J. Long, J.A. Brown, R. Nunes, et al. 2001. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2:261–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous