Orchestrating the unfolded protein response in health and disease - PubMed (original) (raw)
Review
Orchestrating the unfolded protein response in health and disease
Randal J Kaufman. J Clin Invest. 2002 Nov.
No abstract available
Figures
Figure 1
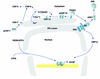
Signaling the UPR in eukaryotes. Three proximal sensors, IRE1, PERK, and ATF6, coordinately regulate the UPR through their various signaling pathways. Whereas IRE1 and PERK are dispensable for many aspects of the response, ATF6 cleavage is required for UPR transcriptional induction and appears to be the most significant of these effectors in mammalian cells. BiP negatively regulates these pathways. BiP interacts with ATF6 to prevent its transport to the Golgi compartment (a). BiP binds to the lumenal domains of IRE1 (b) and PERK (c) to prevent their dimerization. As unfolded proteins accumulate, they bind BiP and reduce the amount of BiP available to bind and inhibit activation of IRE1, PERK, and ATF6. (a) BiP release from ATF6 permits transport to the Golgi compartment. In the Golgi, ATF6 is cleaved by S1P and S2P proteases to yield a cytosolic fragment that migrates to the nucleus to activate transcription of responsive genes, including XBP1. (b) BiP release from IRE1 permits dimerization to activate its kinase and RNase activities to initiate XBP1 mRNA splicing. XBP1 splicing removes a 26-base intron, creating a translational frameshift to yield a more potent transcriptional activator. (c) BiP release permits PERK dimerization and activation to phosphorylate Ser51 on eIF2α to reduce the frequency of AUG initiation codon recognition. As eIF2α phosphorylation reduces the functional level of eIF2, the general rate of translation initiation is reduced. However, selective mRNAs, such as ATF4 mRNA, are preferentially translated under these conditions, possibly by the presence of open reading frames within the 5′ untranslated region of the mRNA. Upon recovery from the UPR, GADD34 targets PP1 to dephosphorylate eIF2α and increase protein translation.
Figure 2
Signaling UPR-mediated cell death. The activation of procaspase-12 is likely the major pathway that induces apoptosis in response to ER stress. Upon activation of the UPR, c-Jun-N-terminal inhibitory kinase (JIK) release from procaspase-12 permits clustering and activation of procaspase-12. Caspase-12 activates procaspase-9 to activate procaspase-3, the executioner of cell death. In addition, activated IRE1 binds JIK and recruits TRAF2, which signals through apoptosis-signaling kinase 1 (ASK1) and JNK to promote mitochondria-dependent apoptosis. In addition, in vitro studies suggest that localized calcium release from the ER activates m-calpain to cleave and activate procaspase-12. Upon UPR activation, procaspase-7 is activated and recruited to the ER membrane. Finally, IRE1, PERK, and ATF6 induce transcription of several genes encoding apoptotic functions, including CHOP/GADD153. CSP, caspase; pCSP, procaspase.
Similar articles
- Human cytomegalovirus infection activates and regulates the unfolded protein response.
Isler JA, Skalet AH, Alwine JC. Isler JA, et al. J Virol. 2005 Jun;79(11):6890-9. doi: 10.1128/JVI.79.11.6890-6899.2005. J Virol. 2005. PMID: 15890928 Free PMC article. - The unfolded protein response.
Liu CY, Kaufman RJ. Liu CY, et al. J Cell Sci. 2003 May 15;116(Pt 10):1861-2. doi: 10.1242/jcs.00408. J Cell Sci. 2003. PMID: 12692187 Review. No abstract available. - [Stress signaling from the endoplasmic reticulum].
Urano F. Urano F. Tanpakushitsu Kakusan Koso. 2004 May;49(7 Suppl):1002-5. Tanpakushitsu Kakusan Koso. 2004. PMID: 15168512 Review. Japanese. No abstract available. - Unfolded protein response.
Cao SS, Kaufman RJ. Cao SS, et al. Curr Biol. 2012 Aug 21;22(16):R622-6. doi: 10.1016/j.cub.2012.07.004. Curr Biol. 2012. PMID: 22917505 No abstract available. - Heat shock protein 90 modulates the unfolded protein response by stabilizing IRE1alpha.
Marcu MG, Doyle M, Bertolotti A, Ron D, Hendershot L, Neckers L. Marcu MG, et al. Mol Cell Biol. 2002 Dec;22(24):8506-13. doi: 10.1128/MCB.22.24.8506-8513.2002. Mol Cell Biol. 2002. PMID: 12446770 Free PMC article.
Cited by
- Glucocorticoids alleviate intestinal ER stress by enhancing protein folding and degradation of misfolded proteins.
Das I, Png CW, Oancea I, Hasnain SZ, Lourie R, Proctor M, Eri RD, Sheng Y, Crane DI, Florin TH, McGuckin MA. Das I, et al. J Exp Med. 2013 Jun 3;210(6):1201-16. doi: 10.1084/jem.20121268. Epub 2013 May 6. J Exp Med. 2013. PMID: 23650437 Free PMC article. - Hsp70 promotes epithelial sodium channel functional expression by increasing its association with coat complex II and its exit from endoplasmic reticulum.
Chanoux RA, Robay A, Shubin CB, Kebler C, Suaud L, Rubenstein RC. Chanoux RA, et al. J Biol Chem. 2012 Jun 1;287(23):19255-65. doi: 10.1074/jbc.M112.357756. Epub 2012 Apr 10. J Biol Chem. 2012. PMID: 22496374 Free PMC article. - Expression and distribution of grp-78/bip in mineralizing tissues and mesenchymal cells.
Ravindran S, Gao Q, Ramachandran A, Sundivakkam P, Tiruppathi C, George A. Ravindran S, et al. Histochem Cell Biol. 2012 Jul;138(1):113-25. doi: 10.1007/s00418-012-0952-1. Epub 2012 Apr 17. Histochem Cell Biol. 2012. PMID: 22527697 Free PMC article. - Roles of endoplasmic reticulum and energetic stress in disturbed sleep.
Naidoo N. Naidoo N. Neuromolecular Med. 2012 Sep;14(3):213-9. doi: 10.1007/s12017-012-8179-9. Epub 2012 Apr 20. Neuromolecular Med. 2012. PMID: 22527792 Review.
References
- Lee AS. Mammalian stress response: induction of the glucose-regulated protein family. Curr Opin Cell Biol. 1992;4:267–273. - PubMed
- Kaufman RJ, et al. The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol. 2002;3:411–421. - PubMed
- Ellgaard L, Helenius A. ER quality control: towards an understanding at the molecular level. Curr Opin Cell Biol. 2001;13:431–437. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources