Endocytosis is a critical step in entry of subgroup B avian leukosis viruses - PubMed (original) (raw)
Endocytosis is a critical step in entry of subgroup B avian leukosis viruses
Felipe Diaz-Griffero et al. J Virol. 2002 Dec.
Abstract
The avian leukosis virus (ALV) entry mechanism is controversial, with evidence for and against a low-pH requirement for viral fusion. To further address this question, we tested the entry of human immunodeficiency virus type 1 (HIV-1) pseudotyped with the envelope protein of subgroup B ALV (ALV-B) in the presence of three different lysosomotropic agents. These lysosomotropic agents were able to block the entry of wild-type and pseudotyped ALV-B in two different cell lines, strongly suggesting that ALV-B requires a low-pH step for entry. ALV-B and pH-dependent Semliki Forest virus (SFV) entered cells with slower uptake kinetics than HIV-1, which is pH independent. These slow uptake rates support the theory that ALV-B utilizes endocytic pathways to enter cells. Using immunofluorescence and electron microscopy analysis, we visualized the colocalization of virus particles with the endosomal marker transferrin and demonstrated virus particles in clathrin-coated vesicles and endosome-like structures. Surprisingly, a low-pH treatment did not overcome the inhibition of ALV-B entry by lysosomotropic agents. This indicates that, in contrast to SFV, ALV-B is unable to fuse at the cellular surface, even at a low pH. Taken together, our findings suggest that endocytosis and a subsequent low-pH step are critical for successful ALV-B infection.
Figures
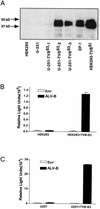
FIG. 1.
Entry assay of an HIV-1-based vector pseudotyped with the envelope protein of ALV-B. (A) Astrocytoma cells (U-251) and HEK 293 cells were stably transfected with TVBS3-expressing vector. Extracts from chicken embryonic fibroblasts (DF-1) and human cell lines transfected with or without TVBS3 were prepared, and 10 μg of protein was analyzed by Western blot analysis to detect TVBS3 expression. (B) HIV-1 vector (NLluc+env−) was pseudotyped with the envelope protein of ALV-B. Parental and HEK 293-TVBS3 cells were infected for 3 h with pseudotyped ALV-B viruses (10 ng of p24). Supernatant was removed and replaced with fresh medium, and luciferase activity was measured 48 h postinfection. (C) Parental and U-251-TVBS3 were infected with pseudotyped ALV-B (10 ng of p24) as described above for panel B. Experiments were performed in triplicates, and standard deviations are indicated for panels B and C.
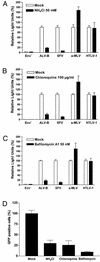
FIG. 2.
Entry of wild-type and pseudotyped ALV-B is inhibited by lysosomotropic agents. HEK 293-TVBS3 cells were incubated with pseudotyped ALV-B, SFV, a-MLV, or HTLV-1 for 4 h in the presence or absence of 50 mM NH4Cl (A), 100 μg of chloroquine/ml (B), or 50 nM bafilomycin _A_1 (C). Cells were challenged with pseudotyped ALV-B, SFV, a-MLV, and HTLV-1 with similar amounts of p24.Luciferase activity was measured from cellular extracts 48 h postinfection. (D) HEK 293-TVBS3 cells were incubated with wild-type ALV-B that expresses GFP (MOI of 10) in the presence or absence of 50 mM NH4Cl, 100 μg of chloroquine/ml, or 50 nM of bafilomycin _A_1 for 4 h. Infection was determined by flow cytometry analysis by counting GFP-positive cells 72 h postinfection. Experiments were performed in triplicate, and standard deviations are indicated.
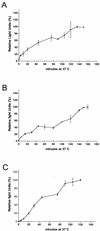
FIG. 3.
Entry kinetics of pseudotyped ALV-B. Pseudotyped ALV-B (10 ng of p24) was prebound to HEK 293-TVBS3 (A) or U-251-TVBS3 (B) cells at 4°C for 1 h. Infection was initiated by shifting the temperature to 37°C. At the indicated time point, cells were incubated for 2 to 3 h with 50 mM NH4Cl to stop viral entry. Subsequently, supernatants were replaced with fresh medium and luciferase activity was determined 48 h postinfection. Pseudotyped SFV was prebound to HEK 293-TVBS3 cells, and entry kinetics were determined as described above (C). Experiments were performed in triplicate, and standard deviations are shown.
FIG. 4.
Pseudotyped ALV-B particles colocalize with an endosomal marker. (A) Pseudotyped ALV-B particles (10 ng of p24) were prebound to U-251-TVBS3-3 cells, and infection was started by shifting cells to 37°C. Cells were processed for immunofluorescence, stained using the anti-p24 antibodies, and developed with a Cy3-labeled antibody (arrows). (B) Pseudotyped ALV-B and human transferrin-Alexa 488 (green) were prebound to U-251-TVBS3-3 cells at 4°C for 1 h. Viral infection and transferrin uptake were initiated by shifting the temperature to 37°C. After 30 min, cells were processed for immunofluorescence with an anti-p24 antibody and developed with a Cy3-labeled antibody (red). Z sections were obtained in both filters (Alexa 488 and Cy3) of a wide-field fluorescence microscope. Images were deconvolved as described in Materials and Methods. The 3-D projection of an infected cell (30 sections) was done in Imaris version 3.0, showing pseudotyped ALV-B particles (red), endosomes (green), and colocalization (yellow). (C) Individual Z sections are shown, and the distance from the cell surface is indicated in micrometers.
FIG. 4.
Pseudotyped ALV-B particles colocalize with an endosomal marker. (A) Pseudotyped ALV-B particles (10 ng of p24) were prebound to U-251-TVBS3-3 cells, and infection was started by shifting cells to 37°C. Cells were processed for immunofluorescence, stained using the anti-p24 antibodies, and developed with a Cy3-labeled antibody (arrows). (B) Pseudotyped ALV-B and human transferrin-Alexa 488 (green) were prebound to U-251-TVBS3-3 cells at 4°C for 1 h. Viral infection and transferrin uptake were initiated by shifting the temperature to 37°C. After 30 min, cells were processed for immunofluorescence with an anti-p24 antibody and developed with a Cy3-labeled antibody (red). Z sections were obtained in both filters (Alexa 488 and Cy3) of a wide-field fluorescence microscope. Images were deconvolved as described in Materials and Methods. The 3-D projection of an infected cell (30 sections) was done in Imaris version 3.0, showing pseudotyped ALV-B particles (red), endosomes (green), and colocalization (yellow). (C) Individual Z sections are shown, and the distance from the cell surface is indicated in micrometers.
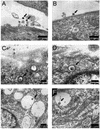
FIG. 5.
Wild-type ALV-B is in clathrin-coated vesicles and endosome-like structures. Wild-type ALV-B viruses were prebound to DF-1 cells at 4°C for 1 h. Infection was initiated by shifting the temperature to 37°C. Thirty minutes after infection was initiated, samples were processed for ultrastructural analysis. Virus particles were observed (arrows) at a magnification of ×12,000 at the surface of DF-1 cells (A and B), in clathrin-coated vesicles (C and D), and in vesicles (E) while fusing to the vesicle membrane (F).
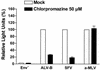
FIG. 6.
Entry of pseudotyped ALV-B is inhibited by chlorpromazine. HEK 293-TVB S3 cells were incubated with pseudotyped ALV-B, SFV, or a-MLV for 3 h in the presence or absence of 50 μM chlorpromazine. Cells were challenged with pseudotyped ALV-B, SFV, or a-MLV by using similar amounts of p24. Luciferase activity was measured from cellular extracts 48 h postinfection. Experiments were performed in triplicate, and standard deviations are shown.
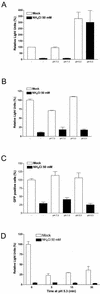
FIG.7.
Wild-type and pseudotyped ALV-B were unable to fuse at the cell surface upon low-pH treatment. Pseudotyped SFV (10 ng of p24) (A), pseudotyped ALV-B (10 ng of p24) (B), and wild-type ALV-B (MOI = 10) (C) were prebound to HEK 293-TVBS3 cells at 4°C for 1 h. Cells were treated with a medium, pH 5.3 or 7.0, for 2 min at 37°C. Subsequently, cells were incubated in fresh medium for 4 h in the presence or absence of 50 mM NH4Cl. Supernatants were replaced with fresh medium, and luciferase activity was measured 48 h postinfection (A and B). Flow cytometry analysis for GFP-positive cells was used to determine infection by wild-type ALV-B (C). Pseudotyped ALV-B (10 ng of p24) was prebound to HEK 293-TVBS3 cells at 4°C for 1 h. Cells were treated with a medium, pH 5.3, containing or lacking 50 mM NH4Cl for 5, 15, or 30 min at 37°C. Subsequently, cells were incubated with medium containing 50 mM NH4Cl for 4 h. Cells were grown in fresh medium, and extracts were prepared 48 h postinfection to measure luciferase activity (D). Experiments were performed in triplicate, and standard deviations are shown.
Similar articles
- Cellular uptake of avian leukosis virus subgroup B is mediated by clathrin.
Diaz-Griffero F, Jackson AP, Brojatsch J. Diaz-Griffero F, et al. Virology. 2005 Jun 20;337(1):45-54. doi: 10.1016/j.virol.2005.02.027. Virology. 2005. PMID: 15914219 - Identification of a cellular receptor for subgroup E avian leukosis virus.
Adkins HB, Brojatsch J, Naughton J, Rolls MM, Pesola JM, Young JA. Adkins HB, et al. Proc Natl Acad Sci U S A. 1997 Oct 14;94(21):11617-22. doi: 10.1073/pnas.94.21.11617. Proc Natl Acad Sci U S A. 1997. PMID: 9326659 Free PMC article. - Role of TSPAN9 in Alphavirus Entry and Early Endosomes.
Stiles KM, Kielian M. Stiles KM, et al. J Virol. 2016 Apr 14;90(9):4289-97. doi: 10.1128/JVI.00018-16. Print 2016 May. J Virol. 2016. PMID: 26865714 Free PMC article. - [Entry process of enveloped viruses to host cells].
Miyauchi K. Miyauchi K. Uirusu. 2009 Dec;59(2):205-13. doi: 10.2222/jsv.59.205. Uirusu. 2009. PMID: 20218329 Review. Japanese. - Entry of animal viruses and macromolecules into cells.
Carrasco L. Carrasco L. FEBS Lett. 1994 Aug 22;350(2-3):151-4. doi: 10.1016/0014-5793(94)00780-2. FEBS Lett. 1994. PMID: 7915239 Review.
Cited by
- A study of low pH-induced refolding of Env of avian sarcoma and leukosis virus into a six-helix bundle.
Markosyan RM, Bates P, Cohen FS, Melikyan GB. Markosyan RM, et al. Biophys J. 2004 Nov;87(5):3291-8. doi: 10.1529/biophysj.104.047696. Epub 2004 Aug 31. Biophys J. 2004. PMID: 15339808 Free PMC article. - Jaagsiekte sheep retrovirus utilizes a pH-dependent endocytosis pathway for entry.
Bertrand P, Côté M, Zheng YM, Albritton LM, Liu SL. Bertrand P, et al. J Virol. 2008 Mar;82(5):2555-9. doi: 10.1128/JVI.01853-07. Epub 2007 Dec 19. J Virol. 2008. PMID: 18094164 Free PMC article. - Effects of chloroquine on viral infections: an old drug against today's diseases?
Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Savarino A, et al. Lancet Infect Dis. 2003 Nov;3(11):722-7. doi: 10.1016/s1473-3099(03)00806-5. Lancet Infect Dis. 2003. PMID: 14592603 Free PMC article. Review. - Human immunodeficiency virus (HIV) infects human arterial smooth muscle cells in vivo and in vitro: implications for the pathogenesis of HIV-mediated vascular disease.
Eugenin EA, Morgello S, Klotman ME, Mosoian A, Lento PA, Berman JW, Schecter AD. Eugenin EA, et al. Am J Pathol. 2008 Apr;172(4):1100-11. doi: 10.2353/ajpath.2008.070457. Epub 2008 Feb 29. Am J Pathol. 2008. PMID: 18310503 Free PMC article. - Analogue discovery of safer alternatives to HCQ and CQ drugs for SAR-CoV-2 by computational design.
Sinha M, Gupta A, Gupta S, Singh P, Pandit S, Chauhan SS, Parthasarathi R. Sinha M, et al. Comput Biol Med. 2021 Mar;130:104222. doi: 10.1016/j.compbiomed.2021.104222. Epub 2021 Jan 20. Comput Biol Med. 2021. PMID: 33535144 Free PMC article.
References
- Andersen, K. B. 1985. The fate of the surface protein gp70 during entry of retrovirus into mouse fibroblasts. Virology 142:112-120. - PubMed
- Andersen, K. B., and B. A. Nexo. 1983. Entry of murine retrovirus into mouse fibroblasts. Virology 125:85-98. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources