Ultrastructural analysis of differentiation in Legionella pneumophila - PubMed (original) (raw)
FIG. 1.
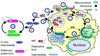
Schematic representation of intracellular and extracellular growth cycles of L. pneumophila. 1, Different L. pneumophila forms may attach to and enter a host cell (protozoan, macrophage, or cell line). Surface-exposed Hsp60, at least in part, mediates attachment and cell entry (28). Mature intracellular forms (MIFs) are often internalized by macrophages through coiling phagocytosis (15, 16). 2, After entry, MIFs and stationary-phase bacteria grown in vitro downregulate flagellar genes (13), inhibit phagosome-lysosome fusion (40), and recruit vesicles and mitochondria around the phagosome (20, 29, 39, 51). Exponential-phase replicative bacteria cannot effectively proceed through this step (40). Synthesis of Hsp60 is upregulated, and this protein is released into the phagosomal space (19). Expression of the Dot/Icm proteins (6, 11, 56, 59) and other factors (40) is required to abolish phagosome-lysosome function and further conditioning of the phagosome. 3, The phagosome associates with the endoplasmic reticulum (ER) (1, 63, 64) to form the replicative compartment. Active replication takes place in ribosome-studded vacuoles. While we have depicted the endoplasmic reticulum and the legionella-containing vacuole as separate entities that remain associated, it has recently been suggested that they form a single continuous compartment (64). The pattern of L. pneumophila protein expression changes (2, 62), and Hsp60 accumulates in the vacuolar space (25). 4, Intermediate forms with a distinct morphology and tinctorial properties arise (27), while replication continues. 5, Termination of intracellular replication leads to full differentiation of MIFs, which acquire a full-virulence phenotype (16, 27, 55) as well as a bright red color after the Gimenez stain and numerous inclusions (16, 26, 27, 29). Flagellum expression and cytotoxicity are upregulated (13), and the abilities to lyse the vacuolar membrane (5) and assemble DotO/H proteins on the bacterial cell surface (65) are induced. 6, MIFs are released to the extracellular medium, either free or inside vesicles. The released bacteria may carry bound host proteins (7) and display unique ultrastructural, physiological, and surface properties (7, 15, 16, 27). 7, MIFs or MIF-packed vesicles (9, 12, 55) may initiate another intracellular cycle. 8, Released MIFs may also start an extracellular growth cycle. Because MIFs do not replicate, differentiation into a replicative intermediate is required (26). 9, Intermediates eventually differentiate into the exponential-phase, actively replicating form (26). 10, In the extracellular growth cycle, L. pneumophila alternates between exponential-phase forms and stationary-phase forms (13). Entry into stationary phase is linked to enhanced virulence and improved resistance to environmental challenges, likely through a complex regulatory network (13, 33). The changing colors of bacteria reflect their tinctorial properties after the Giménez stain. Red is regarded as a Giménez-positive stain, green as a Giménez-negative stain, and purple as a Giménez-intermediate stain (27).
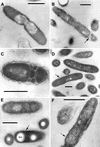
FIG.2.
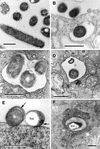
L. pneumophila morphology in the prereplicative phase. (A) Plate-grown legionellae (strain 2064 shown) with a standard gram-negative morphology, used as the inoculum to infect HeLa cells. (B) The overall bacterial morphology did not change during binding or internalization (strain SVir at 6 h postinfection shown). (C) More than one morphologically unchanged bacterium (SVir shown) could colocalize in early vacuoles. (D) Morphologically unchanged bacterium (SVir shown) in a vacuole surrounded by mitochondria (m) and vesicles. (E) HeLa-grown legionellae (SVir shown) tightly bound to the surface of a HeLa cell. Note the unique envelope morphology (multimembranous envelope) (arrows) of the bound bacteria and the prominent inclusion (Inc) in one of them. (F) HeLa-grown legionellae (2064 shown) inside an early vacuole surrounded by a multitude of vesicles. Note the bubble in the bacterial cytoplasmic region produced by a poorly embedded inclusion. Bars, 0.5 μm.
FIG. 3.
L. pneumophila morphology in the early replicative phase. Loose enlarged vacuoles associated with rough endoplasmic reticulum (arrowheads) and containing morphologically unchanged 2064 (A) or SVir (B) bacteria from a plate-grown inoculum, at about 6 h postinfection. (C) At 12 h postinfection, morphologically unchanged SVir bacterial cells from a plate-grown inoculum were often seen individually contained in tight vacuoles. (D) Morphologically unchanged and replicating SVir bacteria from a plate-grown inoculum in tight vacuoles that followed the contour of the enclosed bacteria. (E) Many vacuoles prominently surrounded by ribosomes and/or rough endoplasmic reticulum contained numerous bacteria (SVir shown at 12 h postinfection). Note the vacuolar membrane closely following the contour of the contained bacteria. (F) Bacteria from a HeLa cell-grown inoculum singly contained in tight vacuoles and showing the replicative morphology (strain 2064 at about 20 h postinfection). Bars, 0.5 μm.
FIG.4.
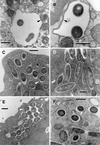
L. pneumophila morphology in late replicative phase. (A) SVir bacteria displaying an electron-dense cytoplasm and an outer membrane with sharp ripples, 25 h postinfection. Note that these morphologically distinct bacteria coexisted with bacteria displaying a standard gram-negative envelope and a less electron-dense cytoplasm. (B) Some bacteria (strain 2064 from a HeLa cell-grown inoculum is shown at 21 h postinfection) developed a rather straight (smooth) outer membrane. The envelope in these bacteria was often difficult to resolve. (C) In the 2064 strain, forms with sharp ripples could be seen at 12 h postinfection but also persisted until later times (D) (arrows), when they coexisted with the smooth-outer-membrane intermediates. (E) Late-replicating bacteria (SVir shown at 25 h postinfection) showed invaginations of the inner membrane (arrowheads). (F) Intricate replicative vacuole containing numerous SVir bacteria with some of the distinctive morphologies described for panels A to E is shown. Bars, 0.5 μm.
FIG. 5.
L. pneumophila morphology in the postreplicative phase. (A) Extended invaginations of the inner membrane that resulted in a multilayered envelope (SVir strain shown at 48 h postinfection). Bar, 0.25 μm. (B) Section of SVir (bacterium found free in the supernatant of infected HeLa cells 3 days postinfection) showing the characteristic thick layer associated with the inner leaflet of the outer membrane (arrow). Postreplicative SVir forms typically had irregular shapes, as shown in specimens fixed with our standard protocol (C) (bar, 0.1 μm) or the Karnovsky fixation protocol (D) (bar, 0.25 μm). Note that the Karnovsky protocol yielded slightly smoother envelopes.
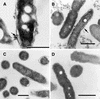
FIG. 6.
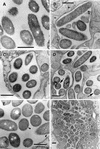
Morphological forms present in purified populations of replicating intracellular legionellae. (A) Intermediate SVir replicative form with sharp ripples in the outer membrane. (B) SVir intermediate with wavy outer membrane and fragmented cytoplasm at one of the poles (arrow). (C) SVir bacterium depicting pronounced fragmentation. The form shown appears to have been fragmented, possibly due to excessive membrane invagination. (D) Intermediate strain 2064 form (arrow) with a very homogeneous appearance and very straight membranes. Note the morphological differences between this form and the neighbor form above it, which has a typical gram-negative envelope ultrastructure. (E) An intermediate SVir form (arrow) with a very straight outer membrane, a difficult-to-resolve inner membrane, and prominent well-defined inclusions (Inc). (F) Replicative 2064 intermediate similar to that shown in panel D but displaying an incipient thick layer (arrow). Bars, 0.5 μm.
FIG. 7.
In vitro intermediates of the MIF-to-replicative-form transition. (A) Strain 2064 intermediate depicting intraperiplasmic vesicles (arrow). (B) An SVir intermediate with several intraperiplasmic vesicles (arrow) is shown side by side with a section of a replicating bacterium depicting a standard gram-negative ultrastructure. (C) Typical replicative 2064 forms derived from MIFs ≈20 h after inoculation into BYE broth. (D) Stationary-phase 2064 intermediate with multiple internal membranes but a generally unresolved envelope. Bars, 0.5 μm.
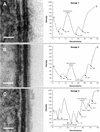
FIG. 8.
Envelope profiles comprising different groups of legionellar forms. Electron micrographs shown on the left side were used to obtain the envelope profiles shown to the right of each panel. (A) Group 1 pattern consisted of the typical gram-negative envelope with inner and outer membranes separated by a periplasmic space. Bar, 20 nm. (B) Group 2 pattern included all the banded envelopes (displaying a thick layer in the inner leaflet of the outer membrane) but no internal cytoplasmic membranes. Bar, 20 nm. (C) Group 3 included all envelopes with internal cytoplasmic membranes with or without a band. The profile shown corresponds to a four-membrane envelope, the most commonly observed in this group. Notice the differences in intensity (peak depths) between the two leaflets of each membrane layer. Bar, 50 nm. (D) Micrograph with no accompanying profile showing a section of an envelope with a total of six membranes, including the outer membrane. Bar, 50 nm. (E) Micrograph with no profile showing a bacterial section with a three-membrane envelope. The central compartment labeled P likely corresponds to an enlarged periplasm. Bar, 100 nm. (F) Group 4 profiles included all envelopes with an unresolved inner membrane. Although the scan showed putative peaks for the inner membrane, they were too shallow, indicating that the intensities of the membrane and surrounding plasma were very similar. Bar, 20 nm. (G) Group 5 represents spore-like envelopes with an electron-dense periplasm and a multilayered appearance. The outer membrane remained unresolved. Bar, 20 nm. OL-IM, outer leaflet of the inner membrane; IL-IM, inner leaflet of the inner membrane; OL-OM, outer leaflet of the outer membrane; IL-OM, inner leaflet of the outer membrane; P, periplasm; C, cytoplasm; E, extracellular space.
FIG. 8.
Envelope profiles comprising different groups of legionellar forms. Electron micrographs shown on the left side were used to obtain the envelope profiles shown to the right of each panel. (A) Group 1 pattern consisted of the typical gram-negative envelope with inner and outer membranes separated by a periplasmic space. Bar, 20 nm. (B) Group 2 pattern included all the banded envelopes (displaying a thick layer in the inner leaflet of the outer membrane) but no internal cytoplasmic membranes. Bar, 20 nm. (C) Group 3 included all envelopes with internal cytoplasmic membranes with or without a band. The profile shown corresponds to a four-membrane envelope, the most commonly observed in this group. Notice the differences in intensity (peak depths) between the two leaflets of each membrane layer. Bar, 50 nm. (D) Micrograph with no accompanying profile showing a section of an envelope with a total of six membranes, including the outer membrane. Bar, 50 nm. (E) Micrograph with no profile showing a bacterial section with a three-membrane envelope. The central compartment labeled P likely corresponds to an enlarged periplasm. Bar, 100 nm. (F) Group 4 profiles included all envelopes with an unresolved inner membrane. Although the scan showed putative peaks for the inner membrane, they were too shallow, indicating that the intensities of the membrane and surrounding plasma were very similar. Bar, 20 nm. (G) Group 5 represents spore-like envelopes with an electron-dense periplasm and a multilayered appearance. The outer membrane remained unresolved. Bar, 20 nm. OL-IM, outer leaflet of the inner membrane; IL-IM, inner leaflet of the inner membrane; OL-OM, outer leaflet of the outer membrane; IL-OM, inner leaflet of the outer membrane; P, periplasm; C, cytoplasm; E, extracellular space.
FIG. 9.
Freeze fracture analysis of agar-grown and postreplicative SVir forms. (A and B) Fracture replicas of MIFs, showing both cytoplasmic inclusions and an uneven distribution of intramembranous particles in the inner membrane. (C) Fracture replicas of agar-grown bacteria. Notice the lack of inclusions in the exposed fractures across the cytoplasm and the even distribution of intramembranous particles in the inner membrane. (D) Fracture replicas of MIFs after they were grown on BCYE plates to form a visible film. It was common to see small vesicles in areas corresponding to the periplasm (arrowheads) and inner membranes with evenly distributed intramembranous particles. Bars, 1.0 μm.