Localized suppression of RhoA activity by Tyr31/118-phosphorylated paxillin in cell adhesion and migration - PubMed (original) (raw)
Localized suppression of RhoA activity by Tyr31/118-phosphorylated paxillin in cell adhesion and migration
Asako Tsubouchi et al. J Cell Biol. 2002.
Abstract
RhoA activity is transiently inhibited at the initial phase of integrin engagement, when Cdc42- and/or Rac1-mediated membrane spreading and ruffling predominantly occur. Paxillin, an integrin-assembly protein, has four major tyrosine phosphorylation sites, and the phosphorylation of Tyr31 and Tyr118 correlates with cell adhesion and migration. We found that mutation of Tyr31/118 caused enhanced activation of RhoA and premature formation of stress fibers with substantial loss of efficient membrane spreading and ruffling in adhesion and migration of NMuMG cells. These phenotypes were similar to those induced by RhoA(G14V) in parental cells, and could be abolished by expression of RhoA(T19N), Rac1(G12V), or p190RhoGAP in the mutant-expressing cells. Phosphorylated Tyr31/118 was found to bind to two src homology (SH)2 domains of p120RasGAP, with coprecipitation of endogenous paxillin with p120RasGAP. p190RhoGAP is known to be a major intracellular binding partner for the p120RasGAP SH2 domains. We found that Tyr31/118-phosphorylated paxillin competes with p190RhoGAP for binding to p120RasGAP, and provides evidence that p190RhoGAP freed from p120RasGAP efficiently suppresses RhoA activity during cell adhesion. We conclude that Tyr31/118-phosphorylated paxillin serves as a template for the localized suppression of RhoA activity and is necessary for efficient membrane spreading and ruffling in adhesion and migration of NMuMG cells.
Figures
Figure 1.
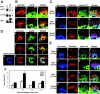
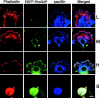
The 2X mutant of paxillin induces phenotypes similar to those by RhoA(G14V) in NMuMG cells. (A) Phosphorylation of Tyr31 and Tyr118 of endogenous paxillin (▴) and the EGFP-tagged 2X mutant (▵) in parental NMuMG cells (P) or the 2X-expressing cells (2X) were visualized using the phosphorylation site-specific antibodies (pY 31 and pY118). Paxillin was detected using an anti-paxillin antibody (pax). (B–E) Formation of stress fibers and membrane ruffles in migration (B, C, and E) or adhesion onto collagen type I (D). NMuMG cells expressing EGFP-tagged wild-type paxillin or the 2X mutant, or HA-tagged RhoA, Rac1, or Cdc42 mutants are shown, as indicated. F-actin was visualized by phalloidin, EGFP-tagged paxillin by autofluorescence of the tag, and Rho GTPases by an anti-HA antibody. Bars, 20 μm. (F) NMuMG cells expressing EGFP-tagged wild-type paxillin (WT) or the 2X mutant (2X) were replated onto collagen coated dishes in the presence of 1% serum, and cell lysates prepared from each time point were incubated with GST-Rhotekin-RBD to measure the activities of RhoA. RhoA activity is indicated as percentages of the amount of RBD-bound RhoA normalized to the amount of RhoA in total cell lysates. Results are means ±SEM from three independent experiments.
Figure 2.
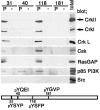
Protein binding to specific paxillin peptides. 500 μg of TGFβ-treated NMuMG cell lysate was incubated with 250 pmol of the phosphorylated (P) and unphosphorylated (−) forms of paxillin peptides immobilized on streptavidin-Sepharose beads, in a total volume of 500 μl for 1 h at 4°C. Proteins precipitated were analyzed by immunoblotting using antibodies as indicated. The numbers indicate the peptides corresponding to the tyrosine phosphorylation sites of paxillin. 10 μg of total cell lysate was included as controls (Total).
Figure 3.
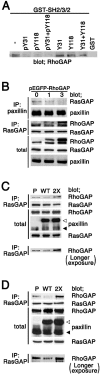
Tyr31/118-phosphorylated paxillin binds to p120RasGAP, via the two SH2 domains. (A) TGFβ-treated (+) or untreated (−) NMuMG cell lysates were immunoprecipitated with a mouse monoclonal anti-paxillin antibody coupled with anti–mouse IgG-Sepharose beads, and subjected to immunoblotting, as indicated. (B and C) TGFβ-treated (+) or untreated (−) NMuMG cell lysates were incubated with GST fusion proteins, each corresponding to various parts of the SH2-SH3-SH2 domain of p120RasGAP (B), and proteins bound to the beads were analyzed by immunoblotting, as indicated (C). Asterisks indicate mutations in the SH2 domains. Coomassie blue staining of the GST proteins used are also shown (CBB). (D) The GST-SH2-SH3-SH2 protein was preincubated with 100 μM each of the phosphorylated or unphosphorylated paxillin peptides (or combinations of peptides), as indicated, before being incubated with TGFβ-treated NMuMG cell lysate, and bound proteins were subjected to anti-paxillin blotting. (E) TGFβ-treated NMuMG cell lysates were incubated with a mouse monoclonal anti-paxillin antibody (IP) or the GST-SH2-SH3-SH2 protein, and bound proteins were analyzed using phosphorylation site-specific antibodies for paxillin (pY31, pY40, pY118 and pY181) or an anti-paxillin antibody, as indicated. 20 pmol each of the paxillin peptides used in Fig. 2 was spotted onto filters and used as controls (right). Total cell lysate (Total) or cell lysate incubated with GST were included as controls, and the molecular sizes in kD are shown, where necessary.
Figure 4.
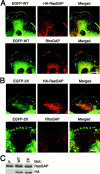
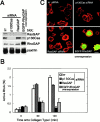
Colocalization of paxillin, p120RasGAP, and p190RhoGAP at the cell periphery, but not at focal adhesions. HA-p120RasGAP was stably expressed at a low level in NMuMG cells expressing EGFP-tagged wild-type paxillin (A) or the 2X mutant (B), and cells were analyzed during migration. EGFP-paxillin was visualized by autofluorescence from the tag. HA-p120RasGAP and endogenous p190RhoGAP were visualized using an anti-HA antibody and an anti-p190RhoGAP antibody, respectively, coupled with Cy5- conjugated anti–mouse IgG. Each right panel is the merged image of the left and middle panels. Bars, 20 μm. Levels of HA-tagged and endogenous p120RasGAP expression in these cells (WT and 2X) are shown by immunoblotting, as indicated (C). Parental NMuMG cells were also included (P).
Figure 5.
Competition of Tyr31/118-phosphorylated paxillin with p190RhoGAP for binding to p120RasGAP. (A) 3Y1/v-Src cell lysates were incubated with the p120RasGAP GST-SH2-SH3-SH2 protein, preincubated with a paxillin peptide (or combinations of peptides) as in Fig. 3 E. The control included the GST fusion protein without incubation with peptides (−). Proteins precipitated were analyzed by anti-p190RhoGAP immunoblotting. (B and C) COS7 cells were transiently transfected with 0, 1, or 3 μg of pEGFP-RhoGAP, as indicated (B), or transfected with 3 μg of pBabe/EGFP-paxillin (WT) or 3 μg of pBabe/EGFP-paxillin 2X (2X), as indicate (C). Mock-transfected cells were also included (C, P). 24 h later, cells were detached from culture dishes by incubation with PBS containing 5 mM EDTA, washed, and replated onto collagen type I–coated dishes in the presence of 0.5% BSA for 1 h. Cells were then lysed, and protein coprecipitation was analyzed using an anti-paxillin antibody coupled with anti–mouse IgG-Sepharose beads (B), or an anti-RasGAP antibody (clone B4F8) coupled with protein A-Sepharose beads (C). Protein immunoblotting was performed as indicated, with immunoprecipitated proteins (IP) and total cell lysates (Total). (D) Amounts of p190RhoGAP coprecipitating with p120RasGAP using antibody B4F8 were analyzed, as above, with cell lysates from parental NMuMG cells (P), or NMuMG cells expressing EGFP-paxillin (WT) or cells expressing the 2X mutant (2X), all pretreated with TGFβ. (C and D) To show the difference in the amounts of p190RhoGAP coprecipitating with p120RasGAP, a longer exposure of the immunoblots shown on the top of each panel, is also shown at the bottom. Exposure time was 15 s for the top, and 2 min for the bottom. (B–D) Total cell lysates were included as controls (Total). Molecular sizes are shown on the left. ▴, endogenous paxillin; ▵, EGFP-tagged paxillin.
Figure 6.
p190RhoGAP overexpression can restore the 2X-induced phenotypes. Confluent cultures of NMuMG cells expressing the 2X mutant were scratched. Cells at the wound edges were microinjected with the pEGFP-p190RhoGAP plasmid, and incubated for 12 h before fixation. Cloned cells stably expressing the paxillin 2X mutant not tagged with EGFP were used, in which expression of the 2X mutant was confirmed using an anti-paxillin and phosphorylation site-specific antibodies as in Fig. 1 A. Mutant and endogenous paxillin were visualized using an anti-paxillin antibody coupled with Cy5-conjugated anti–mouse IgG. F-actin and EGFP-p190RhoGAP were visualized using phalloidin and by the autofluorescence from the tag, respectively. The right panel is the merged image of the left three panels. Cells were classified into four groups, based on the levels of EGFP-RhoGAP expression, which was measured optically from the autofluorescence from the tag. Levels of EGFP-RhoGAP expression were also assessed by staining cells with an anti-RhoGAP antibody and compared with those of endogenous p190RhoGAP in untransfected cells. Levels of EGFP-RhoGAP in each cell of the low level expressors (L) correspond to about the same as that of endogenous p190RhoGAP, the medium (M) correspond to approximately two- to threefold higher, the high (H) correspond to approximately three- to fivefold fold higher, and super high (S) correspond to more than fivefold higher. Representative examples are shown from more than 100 cells examined. Bar, 20 μm.
Figure 7.
Blockage of p120RasGAP expression hampers efficient activation of RhoA in cell adhesion. HeLa cells were treated with 60 nM oligonucleotides in culture medium, designed for the siRNA-mediated specific disruption of p120RasGAP expression or of p130Cas expression, or transfected with pcDNA/EGFP-p190RhoGAP for its overexpression, as indicated. Oligonucleotides with an irrelevant sequence (irr), were included as a negative control. After 24 h, cells were starved of serum for a further 24 h, detached from culture dishes by incubation with PBS containing 5 mM EDTA, and washed and replated onto collagen type I coated dishes in the presence of 1% serum. (A) Protein expression in each cell preparation is shown, by immunoblotting. (B) RhoA activity was measured at each time point, as in Fig. 1 F. Results are means ±SEM from three independent experiments. (C) F-actin was visualized in red, in cells pretreated as indicated and fixed 60 min after adhesion. Expression of EGFP-p190RhoGAP, detected by autofluorescence from the tag, was shown in green. Bar, 20 μm.
Similar articles
- Role of p190RhoGAP in beta 2 integrin regulation of RhoA in human neutrophils.
Dib K, Melander F, Andersson T. Dib K, et al. J Immunol. 2001 May 15;166(10):6311-22. doi: 10.4049/jimmunol.166.10.6311. J Immunol. 2001. PMID: 11342655 - Epithelial cell spreading induced by hepatocyte growth factor influences paxillin protein synthesis and posttranslational modification.
Hopkins AM, Bruewer M, Brown GT, Pineda AA, Ha JJ, Winfree LM, Walsh SV, Babbin BA, Nusrat A. Hopkins AM, et al. Am J Physiol Gastrointest Liver Physiol. 2004 Oct;287(4):G886-98. doi: 10.1152/ajpgi.00065.2004. Epub 2004 Jun 10. Am J Physiol Gastrointest Liver Physiol. 2004. PMID: 15191880 - A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells.
Tomar A, Lim ST, Lim Y, Schlaepfer DD. Tomar A, et al. J Cell Sci. 2009 Jun 1;122(Pt 11):1852-62. doi: 10.1242/jcs.046870. Epub 2009 May 12. J Cell Sci. 2009. PMID: 19435801 Free PMC article. - Paxillin: a focal adhesion-associated adaptor protein.
Schaller MD. Schaller MD. Oncogene. 2001 Oct 1;20(44):6459-72. doi: 10.1038/sj.onc.1204786. Oncogene. 2001. PMID: 11607845 Review. - Paxillin: a crossroad in pathological cell migration.
López-Colomé AM, Lee-Rivera I, Benavides-Hidalgo R, López E. López-Colomé AM, et al. J Hematol Oncol. 2017 Feb 18;10(1):50. doi: 10.1186/s13045-017-0418-y. J Hematol Oncol. 2017. PMID: 28214467 Free PMC article. Review.
Cited by
- The TLK1-MK5 Axis Regulates Motility, Invasion, and Metastasis of Prostate Cancer Cells.
Khalil MI, De Benedetti A. Khalil MI, et al. Cancers (Basel). 2022 Nov 22;14(23):5728. doi: 10.3390/cancers14235728. Cancers (Basel). 2022. PMID: 36497211 Free PMC article. - Stochastic Dynamics of Membrane Protrusion Mediated by the DOCK180/Rac Pathway in Migrating Cells.
Welf ES, Haugh JM. Welf ES, et al. Cell Mol Bioeng. 2010 Mar 1;3(1):30-39. doi: 10.1007/s12195-010-0100-8. Cell Mol Bioeng. 2010. PMID: 20473365 Free PMC article. - Quantitative profiling of spreading-coupled protein tyrosine phosphorylation in migratory cells.
Xie Y, Wang J, Zhang Y, Liu X, Wang X, Liu K, Huang X, Wang Y. Xie Y, et al. Sci Rep. 2016 Aug 24;6:31811. doi: 10.1038/srep31811. Sci Rep. 2016. PMID: 27554326 Free PMC article. - TLK1>Nek1 Axis Promotes Nuclear Retention and Activation of YAP with Implications for Castration-Resistant Prostate Cancer.
Olatunde D, De Benedetti A. Olatunde D, et al. Cancers (Basel). 2024 Aug 22;16(16):2918. doi: 10.3390/cancers16162918. Cancers (Basel). 2024. PMID: 39199688 Free PMC article. - The anaphase-promoting complex/cyclosome activator Cdh1 modulates Rho GTPase by targeting p190 RhoGAP for degradation.
Naoe H, Araki K, Nagano O, Kobayashi Y, Ishizawa J, Chiyoda T, Shimizu T, Yamamura K, Sasaki Y, Saya H, Kuninaka S. Naoe H, et al. Mol Cell Biol. 2010 Aug;30(16):3994-4005. doi: 10.1128/MCB.01358-09. Epub 2010 Jun 7. Mol Cell Biol. 2010. PMID: 20530197 Free PMC article.
References
- Arthur, W.T., L.A. Petch, and K. Burridge. 2000. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10:719–722. - PubMed
- Barry, S.T., H.M. Flinn, M.J. Humphries, D.R. Critchley, and A.J. Ridley. 1997. Requirement for Rho in integrin signalling. Cell Adhes. Commun. 4:387–398. - PubMed
- Bryant, S.S., S. Briggs, T.E. Smithgall, G.A. Martin, F. McCormick, J.H. Chang, S.J. Parsons, and R. Jove. 1995. Two SH2 domains of p120 Ras GTPase-activating protein bind synergistically to tyrosine phosphorylated p190 Rho GTPase-activating protein. J. Biol. Chem. 270:17947–17952. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous