Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages - PubMed (original) (raw)
Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages
Ane Knarreborg et al. Appl Environ Microbiol. 2002 Dec.
Abstract
The effect of dietary fat source (soy oil or a mixture of lard and tallow) and dietary supplementation with antibiotics (a combination of avilamycin at 10 mg kg of feed(-1) and salinomycin at 40 mg kg of feed(-1)) on the bacterial community in the ileum of broiler chickens at different ages (7, 14, 21, and 35 days) was studied using PCR with denaturing gradient gel electrophoresis (DGGE) analysis and bacteriological culture. The bacterial origin of fragments in DGGE profiles was identified by sequencing. Bacterial enumeration results, together with PCR-DGGE profiles, showed that the composition of the microflora was age dependent and influenced by dietary fat source and antibiotic supplementation. An increased incidence of streptococci, enterobacteria, and Clostridium perfringens with age of the chickens was demonstrated. Lactobacilli and C. perfringens were the bacterial groups most strongly affected by the dietary treatments. Moreover, different strains (clonal variants of the alpha-toxin gene) of C. perfringens type A were detected in response to age, dietary fat source, and dietary supplementation with antibiotics.
Figures
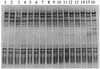
FIG. 1.
PCR-DGGE profiles (30 to 55% denaturing gradient gel) generated from ileal template DNA from 7-day-old chickens, using primer pair HDA1-GC plus HDA2. Lanes 1 to 8 are from replicate pooled samples from 16 chickens fed the S− diet, and lanes 9 to 16 are from replicate pooled samples from 16 chickens fed the S+ diet. Note the consistency of profiles detected in birds of the same age.
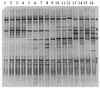
FIG. 2.
PCR-DGGE profiles (30 to 55% denaturing gradient gel) generated from representative ileal content pools, using primer pair HDA1-GC plus HDA2. Lanes: 1 to 4, samples from 7-day-old chickens fed the A− diet (lane 1), the A+ diet (lane 2), the S− diet (lane 3), and the S+ diet (lane 4); 5 to 8, samples from 14-day-old chickens fed the A− diet (lane 5), the A+ diet (lane 6), the S− diet (lane 7), and the S+ diet (lane 8); 9 to 12, samples from 21-day-old chickens fed the A− diet (lane 9), the A+ diet (lane 10), the S− diet (lane 11), and the S+ diet (lane 12); 13 to 16, samples from 35-day-old chickens fed the A− diet (lane 13), the A+ diet (lane 14), the S− diet (lane 15), and the S+ diet (lane 16). The fragments were allotted by sequence analysis to the following genera or species: fragment a, L. johnsonii; fragment b, L. crispatus; fragment c, L. salivarius; fragment d, Streptococcus spp.; fragment e, sequence similarity of 98% to an uncultured bacterium and 97% to L. reuteri; fragment f, C. perfringens.
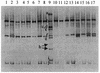
FIG. 3.
Detection of lactobacilli in representative ileal content pools, using primer pair Lac1 plus Lac2-GC in a 30 to 45% gradient gel. Lanes: 1 to 4, samples from 7-day-old chickens fed the A− diet (lane 1), the A+ diet (lane 2), the S− diet (lane 3), and the S+ diet (lane 4); 5 to 8, samples from 14-day-old chickens fed the A− diet (lane 5), the A+ diet (lane 6), the S− diet (lane 7), and the S+ diet (lane 8); 9, identification ladder composed of sequences from reference strains of Lactobacillus species (a, L. plantarum; b, L. johnsonii; c, Weissella viridescens; d, L. gasseri; e, L. acidophilus; f, L. crispatus; g, L. salivarius; h, L. ruminis; i, L. rhamnosus; j, L. reuteri); 10 to 13, samples from 21-day-old chickens fed the A− diet (lane 10), the A+ diet (lane 11), the S− diet (lane 12), and the S+ diet (lane 13); 14 to 17, samples from 35-day-old chickens fed the A− diet (lane 14), the A+ diet (lane 15), the S− diet (lane 16), and the S+ diet (lane 17).
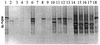
FIG. 4.
Detection of C. perfringens in ileal content pools, using primer pair Cpa1-GC plus Cpa2 in a 20 to 35% gradient gel. Lanes: 1, C. perfringens ATCC 13124T; 2 to 5, samples from 7-day-old chickens fed the A− diet (lane 2), the A+ diet (lane 3), the S− diet (lane 4), and the S+ diet (lane 5); 6 to 9, samples from 14-day-old chickens fed the A− diet (lane 6), the A+ diet (lane 7), the S− diet (lane 8), and the S+ diet (lane 9); 10 to 13, samples from 21-day-old chickens fed the A− diet (lane 10), the A+ diet (lane 11), the S− diet (lane 12), and the S+ diet (lane 13); 14 to 17, samples from 35-day-old chickens fed the A− diet (lane 14), the A+ diet (lane 15), the S− diet (lane 16), and the S+ diet (lane 17); 18, C. perfringens ATCC 13124T. Fragments a (AF477010), b (AF477008), c (AF475144), and d (AF477009) represent four different strains of C. perfringens type A.
Similar articles
- Influence of dietary avilamycin on ileal and cecal microbiota in broiler chickens.
Choi JH, Lee K, Kim DW, Kil DY, Kim GB, Cha CJ. Choi JH, et al. Poult Sci. 2018 Mar 1;97(3):970-979. doi: 10.3382/ps/pex360. Poult Sci. 2018. PMID: 29253227 - Clostridium perfringens challenge and dietary fat type affect broiler chicken performance and fermentation in the gastrointestinal tract.
Józefiak D, Kierończyk B, Rawski M, Hejdysz M, Rutkowski A, Engberg RM, Højberg O. Józefiak D, et al. Animal. 2014 Jun;8(6):912-22. doi: 10.1017/S1751731114000536. Epub 2014 Mar 27. Animal. 2014. PMID: 24674938 - Molecular analysis of intestinal bacterial microbiota of broiler chickens fed diets containing fermented cottonseed meal.
Sun H, Tang JW, Fang CL, Yao XH, Wu YF, Wang X, Feng J. Sun H, et al. Poult Sci. 2013 Feb;92(2):392-401. doi: 10.3382/ps.2012-02533. Poult Sci. 2013. PMID: 23300306 Clinical Trial. - Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens.
Rehman HU, Vahjen W, Awad WA, Zentek J. Rehman HU, et al. Arch Anim Nutr. 2007 Oct;61(5):319-35. doi: 10.1080/17450390701556817. Arch Anim Nutr. 2007. PMID: 18030916 Review.
Cited by
- Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses.
Mohd Shaufi MA, Sieo CC, Chong CW, Gan HM, Ho YW. Mohd Shaufi MA, et al. Gut Pathog. 2015 Feb 26;7:4. doi: 10.1186/s13099-015-0051-7. eCollection 2015. Gut Pathog. 2015. PMID: 25806087 Free PMC article. - Role of Physiology, Immunity, Microbiota, and Infectious Diseases in the Gut Health of Poultry.
Wickramasuriya SS, Park I, Lee K, Lee Y, Kim WH, Nam H, Lillehoj HS. Wickramasuriya SS, et al. Vaccines (Basel). 2022 Jan 22;10(2):172. doi: 10.3390/vaccines10020172. Vaccines (Basel). 2022. PMID: 35214631 Free PMC article. Review. - Impact of feed supplementation with antimicrobial agents on growth performance of broiler chickens, Clostridium perfringens and enterococcus counts, and antibiotic resistance phenotypes and distribution of antimicrobial resistance determinants in Escherichia coli isolates.
Diarra MS, Silversides FG, Diarrassouba F, Pritchard J, Masson L, Brousseau R, Bonnet C, Delaquis P, Bach S, Skura BJ, Topp E. Diarra MS, et al. Appl Environ Microbiol. 2007 Oct;73(20):6566-76. doi: 10.1128/AEM.01086-07. Epub 2007 Sep 7. Appl Environ Microbiol. 2007. PMID: 17827305 Free PMC article. - Effect of Dietary Exogenous Enzyme Supplementation on Enteric Mucosal Morphological Development and Adherent Mucin Thickness in Turkeys.
Ayoola AA, Malheiros RD, Grimes JL, Ferket PR. Ayoola AA, et al. Front Vet Sci. 2015 Oct 13;2:45. doi: 10.3389/fvets.2015.00045. eCollection 2015. Front Vet Sci. 2015. PMID: 26664972 Free PMC article. - Diet-induced changes in the jejunal microbiota of developing broilers reduce the abundance of Enterococcus hirae and Enterococcus faecium.
Stege PB, Schokker D, Harders F, Kar SK, Stockhofe N, Perricone V, Rebel JMJ, de Jong IC, Bossers A. Stege PB, et al. BMC Genomics. 2024 Jun 23;25(1):627. doi: 10.1186/s12864-024-10496-8. BMC Genomics. 2024. PMID: 38910254 Free PMC article.
References
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
- Barnes, E. M., G. C. Mead, and D. A. Barnum. 1972. The intestinal flora of the chicken in the period 2 to 6 weeks of age, with particular reference to the anaerobic bacteria. Br. Poult. Sci. 13:311-326. - PubMed
- Coates, M. E., R. Fuller, G. F. Harrison, M. Lev, and S. F. Suffolk. 1963. A comparison of the growth of chicks in the Gustafsson germ-free apparatus and in a conventional environment with and without dietary supplement of penicillin. Br. J. Nutr. 17:141-150. - PubMed
- Cole, C. B., and R. Fuller. 1984. Bile acid deconjugation and attachment of chicken gut bacteria: their possible role in growth depression. Br. Poult. Sci. 25:227-231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical