Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons - PubMed (original) (raw)
Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons
Nathaniel T Blair et al. J Neurosci. 2002.
Abstract
Nociceptive sensory neurons are unusual in expressing voltage-gated inward currents carried by sodium channels resistant to block by tetrodotoxin (TTX) as well as currents carried by conventional TTX-sensitive sodium channels and voltage-dependent calcium channels. To examine how currents carried by each of these helps to shape the action potential in small-diameter dorsal root ganglion cell bodies, we voltage clamped cells by using the action potential recorded from each cell as the command voltage. Using intracellular solutions of physiological ionic composition, we isolated individual components of current flowing during the action potential with the use of channel blockers (TTX for TTX-sensitive sodium currents and a mixture of calcium channel blockers for calcium currents) and ionic substitution (TTX-resistant current measured by the replacement of extracellular sodium by N-methyl-D-glucamine in the presence of TTX, with correction for altered driving force). TTX-resistant sodium channels activated quickly enough to carry the largest inward charge during the upstroke of the nociceptor action potential (approximately 58%), with TTX-sensitive sodium channels also contributing significantly ( approximately 40%), especially near threshold, and high voltage-activated calcium currents much less (approximately 2%). Action potentials had a prominent shoulder during the falling phase, characteristic of nociceptive neurons. TTX-resistant sodium channels did not inactivate completely during the action potential and carried the majority (58%) of inward current flowing during the shoulder, with high voltage-activated calcium current also contributing significantly (39%). Unlike calcium current, TTX-resistant sodium current is not accompanied by opposing calcium-activated potassium current and may provide an effective mechanism by which the duration of action potentials (and consequently calcium entry) can be regulated.
Figures
Fig. 1.
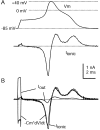
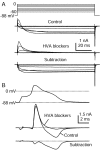
Action potential clamp in small DRG neurons.A, Top, Action potential elicited in a DRG neuron by a short current injection (1.5 nA for 0.5 msec; timing is the same as I_out signal in_B). A, Bottom, Ionic current recorded in voltage clamp with the use of the action potential as a command waveform. Capacity current was eliminated by amplifier circuitry. Holding potential was set to the recorded resting potential of the cell of −85 mV. B, Comparison of ionic current in voltage clamp (black) and the ionic current calculated by scaling the time derivative of the action potential by the measured cell capacitance, 18.7 pF (gray). Also shown is the _I_out signal recorded during the action potential. The action potential was recorded in fast current-clamp mode with bridge balance for electrode series resistance. The dotted line shows zero current level. Internal solution contained (in m
m
): 140 K-Mes, 13.5 NaCl, 1.6 MgCl2, 0.09 EGTA, 9 HEPES, 0.9 glucose, 14 Tris-creatine PO4, 4 Mg-ATP, and 0.3 Tris-GTP, pH 7.2 (with KOH). External solution was normal Tyrode's solution.
Fig. 2.
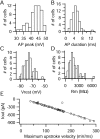
Statistical properties of action potentials in small DRG cells. A–D, Amplitude histograms of action potential peak, action potential duration (measured at half-maximal amplitude), resting potential (measured as average of 3–10 sec), and input resistance. E, Relationship between the maximal negative _I_out signal versus the maximal upstroke velocity. The dotted line shows the least-squares regression line, with a slope of −3 pA per mV/msec and a_y_-intercept of +48 pA.
Fig. 3.
TTX-sensitive and TTX-resistant voltage-gated sodium currents in DRG neurons. A, Currents elicited by 100 msec steps from a holding potential of −80 mV to voltages between −70 and +10 mV in 10 mV increments. Internal solution contained (in m
m
): 120 TEA-Cl, 15 NaCl, 1.8 MgCl2, 9 EGTA, 9 HEPES, 14 Tris-creatine PO4, 4 Mg-ATP, and 0.3 Tris-GTP, pH 7.4. External solution was Tyrode's solution with 30 μ
m
CdCl2 and 5 m
m
TEA-Cl.A, Top, Control. A,Middle, After the addition of 300 n
m
TTX.A, Bottom, TTX-sensitive current obtained by subtraction. B, Currents (same as in_A_) elicited during −20 mV step in control, 300 n
m
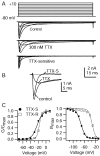
TTX, and resulting subtraction (TTX-S) shown on an expanded time base. The dotted line shows zero current level. C, Left, Voltage dependence of peak conductance for TTX-S (filled circles) and TTX-R (open squares) sodium currents (same cell as in A). Conductance (G) was calculated as G =I/(V −_V_rev), in which I is the peak current, V is the voltage, and_V_rev is the reversal potential for sodium channel current (taken as +58 mV). G is plotted normalized to G_max, the peak conductance for a step to +10 mV. Filled circles, TTX-S current; open squares, TTX-R current. The_lines are best fits to the Boltzmann function:1/(1+exp[−(V−V½)/k]),where V is the step membrane potential in millivolts, _V_1/2 is the half-maximal voltage in millivolts, and k is the slope factor in millivolts. TTX-S (solid line), _V_1/2= −22.8 mV and k = 6.9 mV; TTX-R (dotted line), V_1/2 = −17.3 mV and_k = 3.4 mV. C, Right, Voltage dependence of inactivation determined by using 500 msec prepulses and test pulses to −10 mV. Test pulse current is normalized to its maximal value. Solid curves are best fits to the Boltzmann function:1/(1+exp[(V−V½)/k]),where V is the prepulse membrane potential, _V_1/2 is the half-maximal voltage, and k is the slope factor in millivolts. TTX-S (solid line), _V_1/2 = −72.3 mV and k = 8.2 mV; TTX-R (dotted line), _V_1/2 = −32.4 mV and k = 6.1 mV.
Fig. 4.
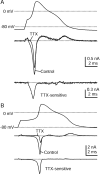
Time course of TTX-S sodium current during the action potential as measured with physiological solutions.A, Top, Action potential recorded from a DRG neuron 23 μm in diameter. A,Middle, Currents (single sweeps) recorded during action potential clamp in control Tyrode's solution (black) and after the addition of 300 n
m
TTX (gray). The dotted line indicates zero current level. A,Bottom, TTX-S current derived by subtraction of traces before and after TTX, shown at an increased resolution.B, Currents recorded in a cell 29 μm in diameter that had a larger TTX-S sodium current. Currents are averages of three sweeps. Internal solution contained (in m
m
): 140 K-Mes, 13.5 NaCl, 1.6 MgCl2, 0.09 EGTA, 9 HEPES, 0.9 glucose, 14 Tris-creatine PO4, 4 Mg-ATP, and 0.3 Tris-GTP, pH 7.2 (with KOH). External solution was Tyrode's solution with or without 300 n
m
TTX.
Fig. 5.
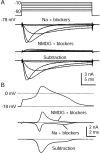
TTX-R sodium current isolated by using NMDG substitution. A, Currents in response to voltage steps between −60 and −10 mV, recorded in Tyrode's solution with 300 n
m
TTX, 5 m
m
TEAC-l, 10 μ
m
nimodipine, 1 μ
m
ω-conotoxin-GVIA, and 250 n
m
ω-agatoxin-IVA (top). Shown are currents recorded after Na+ was replaced completely by NMDG+ (middle) and the resulting subtraction showing isolated TTX-R sodium current (bottom). B, Same current isolation procedure used during the action potential clamp in a 27 μm cell. Shown are action potential waveform (top), currents in Na and NMDG with blockers (middle), and a subtraction yielding raw TTX-R sodium current (bottom). Currents are averages of three sweeps. Na + blockers solution was Tyrode's solution with 300 n
m
TTX, 5 m
m
TEA-Cl, 10 μ
m
nimodipine, 1 μm ω-conotoxin-GVIA, and 250 n
m
ω-Aga-IVA. NMDG + blockers was identical but with NMDG-Cl completely replacing NaCl. Internal solution contained (in m
m
): 140 K-Mes, 13.5 NaCl, 1.6 MgCl2, 0.09 EGTA, 9 HEPES, 0.9 glucose, 14 Tris-creatine PO4, 4 Mg-ATP, and 0.3 Tris-GTP, pH 7.2 (with KOH).
Fig. 6.
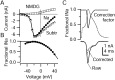
Correction process for NMDG subtraction method.A, Peak sodium current as a function of voltage, recorded in Na Tyrode's solution (filled circles) or in NMDG Tyrode's solution (open squares), each with 30 μ
m
CdCl2 and 5 m
m
TEA added. Internal solution was designed to block potassium currents completely (in m
m
): 120 TEA-Cl, 15 NaCl, 1.8 MgCl2, 9 EGTA, 9 HEPES, 14 Tris-creatine PO4, 4 Mg-ATP, and 0.3 Tris-GTP, pH 7.4. Open triangles show the subtraction current. B, Ratio of current in Na Tyrode's solution (true sodium current) to the current obtained by NMDG subtraction (too big because of outward sodium current after NMDG substitution). The correction factor (solid line) was generated by curve fitting and extrapolating, extending the calculated value at −25 mV (0.98) to −80 mV and extending a straight line from +35 to +50 mV. C,Top, Correction factor during action potential.C, Bottom, Raw (solid line) and corrected (dashed line) TTX-R sodium currents obtained from NMDG subtraction. Traces in C are from the same cell as in Figure 5.
Fig. 7.
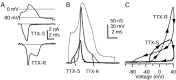
High resolution recording of TTX-S and TTX-R sodium currents flowing during the action potential with the use of internal solution to block potassium currents. Experiments used a previously recorded action potential from a different cell.A, TTX-S and TTX-R currents elicited by the action potential waveform. TTX-S current was calculated as a current blocked by 300 n
m
TTX, averaged over three sweeps. Internal solution contained (in m
m
): 130 NMDG, 120 aspartate, 15 NaCl, 1.8 MgCl2, 9 EGTA, 9 HEPES, 14 Tris-creatine PO4, 4 Mg-ATP, and 0.3 Tris-GTP, pH 7.4 (with 7 m
m
CsOH). External solution was (in m
m
): 150 NaCl, 4 CsCl, 2 BaCl2, 0.3 CdCl2, 10 glucose, and 10 HEPES, pH 7.4. TTX-R sodium current was recorded in a different cell; leak and capacity current was removed by subtraction of the appropriately scaled current elicited by a scaled (0.2), inverted action potential. Shown is an average of two sweeps, digitally filtered at 5 kHz. The dotted lines indicate zero current level. External solution contained (in m
m
): 150 NaCl, 4 CsCl, 2 CaCl2, 2 MgCl2, 0.03 CdCl2, 5 TEA-Cl, 10 glucose, and 10 HEPES, pH 7.4, with 300 n
m
TTX. B, Time course of TTX-S and TTX-R sodium conductances during the action potential, calculated by dividing the currents recorded in A by the driving force on sodium, assuming a sodium equilibrium potential of +60 mV. C, TTX-S and TTX-R sodium conductances from_B_ plotted against voltage during the action potential. Vertical scale is the same as in B.
Fig. 8.
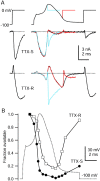
Time course of inactivation of TTX-S and TTX-R sodium currents during the action potential. A,Top, Three command waveforms consisting of varying durations of a typical action potential (same as Fig. 7) preceding a test step to 0 mV. A, Middle, TTX-S sodium current. A, Bottom, TTX-R sodium current (note that outward currents during the peak of the action potential are outward sodium currents resulting from reduced external sodium concentration). For comparison, TTX-S and TTX-R sodium currents elicited during a step from −100 to 0 mV are shown at_left_. External solution for TTX-S current recording was (in m
m
): 50 NaCl, 100 TEA-Cl, 2 BaCl2, 0.3 CdCl2, 10 glucose, and 10 HEPES, pH 7.4, and for TTX-R current recording was (in m
m
): 50 NaCl, 100 TEA-Cl, 4 CsCl, 2 CaCl2, 2 MgCl2, 0.03 CdCl2, 10 glucose, and 10 HEPES, pH 7.4, with 300 n
m
TTX. Internal solution for both contained (in m
m
): 130 NMDG, 120 aspartate, 15 NaCl, 1.8 MgCl2, 9 EGTA, 9 HEPES, 14 Tris-creatine PO4, 4 Mg-ATP, and 0.3 Tris-GTP, pH 7.4 (with 7 m
m
CsOH). B, Time course of TTX-S and TTX-R sodium current availability changes during an action potential. Sodium current was measured during the 0 mV test step, reflecting both channels closed but available to being open and also channels already open at the beginning of the test pulse (the two together constituting “available” channels). Test pulse current was normalized to the first test step current and was plotted against the time of test step onset. TTX-S current amplitude was measured 0.62 msec after the initiation of the 0 mV test step; TTX-R current was measured 1.75 msec after test step initiation.
Fig. 9.
HVA calcium currents elicited by action potential clamp in small DRG cells. A, Currents elicited by step depolarizations in control (top) and then after the application of 10 μ
m
nimodipine, 1 μ
m
ω-conotoxin-GVIA, and 250 n
m
ω-Aga-IVA (middle). Internal solution contained (in m
m
): 140 K-Mes, 13.5 NaCl, 1.6 MgCl2, 0.09 EGTA, 9 HEPES, 0.9 glucose, 14 Tris-creatine PO4, 4 Mg-ATP, and 0.3 Tris-GTP, pH 7.2 (with KOH). External solution for initial recording of action potential was Tyrode's solution. External solution for recording calcium current contained (in m
m
): 160 TEA-Cl, 2 CaCl2, 2 MgCl2, and 10 HEPES, pH 7.4. Note outward Na current exiting through TTX-R sodium channels. Subtraction of currents with and without blockers yields the HVA calcium current (bottom). In these traces 120 μsec after the voltage step has been blanked to remove uncompensated capacitive current.B, Subtraction procedure during action potential clamp (same cell as in A). Currents recorded in 160 TEA-Cl external solution (black) and in the presence of HVA blocker mixture (gray) are shown in the_middle_. The resulting subtraction (bottom) shows that most HVA calcium current flows during the shoulder in the action potential. Currents are the average of three sweeps.
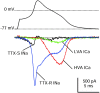
Fig. 10.
Comparison of different inward currents during action potential clamp recorded in a single DRG neuron (same as Fig.5). The action potential was recorded and used as command potential, and TTX-S sodium (black), TTX-R sodium (blue), HVA calcium (red), and LVA calcium (green) currents were isolated as detailed in Results. This cell had a resting potential of −86 mV when averaged over a longer duration before the action potential was recorded. Internal solution contained (in m
m
): 140 K-Mes, 13.5 NaCl, 1.6 MgCl2, 0.09 EGTA, 9 HEPES, 0.9 glucose, 14 Tris-creatine PO4, 4 Mg-ATP, and 0.3 Tris-GTP, pH 7.2 (with KOH).
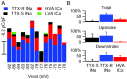
Fig. 11.
Contributions of the major inward currents to small DRG cell action potentials. A, The relative amplitudes of overall charge transferred by each conductance, recorded in the same cell, are plotted against the resting potential of the cell. An anomalous cell expressing purely TTX-S current is obvious (−77 mV resting potential). The latencies of the particular action potentials used for this analysis varied from 1.1 to 11.8 msec in different cells, and there was no significant difference in action potential parameters when action potentials were elicited in the same cell with different latencies in this range. B, Average normalized charge contributions by the TTX-S sodium, TTX-R sodium, and the HVA calcium current are shown for the overall duration of the action potential and separately for current flowing during the upstroke and downstroke. Error bars indicate SD.
Similar articles
- Analysis of the variation in use-dependent inactivation of high-threshold tetrodotoxin-resistant sodium currents recorded from rat sensory neurons.
Tripathi PK, Trujillo L, Cardenas CA, Cardenas CG, de Armendi AJ, Scroggs RS. Tripathi PK, et al. Neuroscience. 2006 Dec 28;143(4):923-38. doi: 10.1016/j.neuroscience.2006.08.052. Epub 2006 Oct 4. Neuroscience. 2006. PMID: 17027172 - Functional tetrodotoxin-resistant Na(+) channels are expressed presynaptically in rat dorsal root ganglia neurons.
Medvedeva YV, Kim MS, Schnizler K, Usachev YM. Medvedeva YV, et al. Neuroscience. 2009 Mar 17;159(2):559-69. doi: 10.1016/j.neuroscience.2008.12.029. Epub 2008 Dec 30. Neuroscience. 2009. PMID: 19162133 - Celecoxib inhibits Na+ currents in rat dorsal root ganglion neurons.
Park SY, Kim TH, Kim HI, Shin YK, Lee CS, Park M, Song JH. Park SY, et al. Brain Res. 2007 May 7;1148:53-61. doi: 10.1016/j.brainres.2007.02.023. Epub 2007 Feb 21. Brain Res. 2007. PMID: 17359944 - Tetrodotoxin-resistant sodium channels.
Yoshida S. Yoshida S. Cell Mol Neurobiol. 1994 Jun;14(3):227-44. doi: 10.1007/BF02088322. Cell Mol Neurobiol. 1994. PMID: 7712513 Free PMC article. Review. - Structural Basis for Pharmacology of Voltage-Gated Sodium and Calcium Channels.
Catterall WA, Swanson TM. Catterall WA, et al. Mol Pharmacol. 2015 Jul;88(1):141-50. doi: 10.1124/mol.114.097659. Epub 2015 Apr 6. Mol Pharmacol. 2015. PMID: 25848093 Free PMC article. Review.
Cited by
- Nicotine suppresses hyperexcitability of colonic sensory neurons and visceral hypersensivity in mouse model of colonic inflammation.
Abdrakhmanova GR, Kang M, Imad Damaj M, Akbarali HI. Abdrakhmanova GR, et al. Am J Physiol Gastrointest Liver Physiol. 2012 Apr;302(7):G740-7. doi: 10.1152/ajpgi.00411.2011. Epub 2012 Jan 12. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22241859 Free PMC article. - Peripheral drive in Aα/β-fiber neurons is altered in a rat model of osteoarthritis: changes in following frequency and recovery from inactivation.
Wu Q, Henry JL. Wu Q, et al. J Pain Res. 2013 Mar 19;6:207-21. doi: 10.2147/JPR.S40445. Print 2013. J Pain Res. 2013. PMID: 23671396 Free PMC article. - Pharmacological dissection and distribution of NaN/Nav1.9, T-type Ca2+ currents, and mechanically activated cation currents in different populations of DRG neurons.
Coste B, Crest M, Delmas P. Coste B, et al. J Gen Physiol. 2007 Jan;129(1):57-77. doi: 10.1085/jgp.200609665. J Gen Physiol. 2007. PMID: 17190903 Free PMC article. - Preferential activation of small cutaneous fibers through small pin electrode also depends on the shape of a long duration electrical current.
Hugosdottir R, Mørch CD, Andersen OK, Helgason T, Arendt-Nielsen L. Hugosdottir R, et al. BMC Neurosci. 2019 Sep 14;20(1):48. doi: 10.1186/s12868-019-0530-8. BMC Neurosci. 2019. PMID: 31521103 Free PMC article. - The role of Nav1.7 in human nociceptors: insights from human induced pluripotent stem cell-derived sensory neurons of erythromelalgia patients.
Meents JE, Bressan E, Sontag S, Foerster A, Hautvast P, Rösseler C, Hampl M, Schüler H, Goetzke R, Le TKC, Kleggetveit IP, Le Cann K, Kerth C, Rush AM, Rogers M, Kohl Z, Schmelz M, Wagner W, Jørum E, Namer B, Winner B, Zenke M, Lampert A. Meents JE, et al. Pain. 2019 Jun;160(6):1327-1341. doi: 10.1097/j.pain.0000000000001511. Pain. 2019. PMID: 30720580 Free PMC article.
References
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. - PubMed
- Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci. 2000;15:331–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS36855/NS/NINDS NIH HHS/United States
- HL35034/HL/NHLBI NIH HHS/United States
- NS38312/NS/NINDS NIH HHS/United States
- R01 NS036855/NS/NINDS NIH HHS/United States
- R37 NS036855/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous