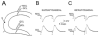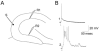Structural and functional asymmetry in the normal and epileptic rat dentate gyrus - PubMed (original) (raw)
Structural and functional asymmetry in the normal and epileptic rat dentate gyrus
Helen E Scharfman et al. J Comp Neurol. 2002.
Abstract
The rat dentate gyrus is usually described as relatively homogeneous. Here, we present anatomic and physiological data which demonstrate that there are striking differences between the supra- and infrapyramidal blades after status epilepticus and recurrent seizures. These differences appear to be an accentuation of a subtle asymmetry present in normal rats. In both pilocarpine and kainic acid models, there was greater mossy fiber sprouting in the infrapyramidal blade. This occurred primarily in the middle third of the hippocampus. Asymmetric sprouting was evident both with Timm stain as well as antisera to brain-derived neurotrophic factor (BDNF) or neuropeptide Y (NPY). In addition, surviving NPY-immunoreactive hilar neurons were distributed preferentially in the suprapyramidal region of the hilus. Extracellular recordings from infrapyramidal sites in hippocampal slices of pilocarpine-treated rats showed larger population spikes and weaker paired-pulse inhibition in response to perforant path stimulation relative to suprapyramidal recordings. A single stimulus could evoke burst discharges in infrapyramidal granule cells but not suprapyramidal blade neurons. BDNF exposure led to spontaneous epileptiform discharges that were larger in amplitude and longer lasting in the infrapyramidal blade. Stimulation of the infrapyramidal molecular layer evoked larger responses in area CA3 than suprapyramidal stimulation. In slices from the temporal pole, in which anatomic evidence of asymmetry waned, there was little evidence of physiological asymmetry either. Of interest, some normal rats also showed signs of greater evoked responses in the infrapyramidal blade, and this could be detected with both microelectrode recording and optical imaging techniques. Although there were no signs of hyperexcitability in normal rats, the data suggest that there is some asymmetry in the normal dentate gyrus and this asymmetry is enhanced by seizures. Taken together, the results suggest that supra- and infrapyramidal blades of the dentate gyrus could have different circuit functions and that the infrapyramidal blade may play a greater role in activating the hippocampus.
Copyright 2002 Wiley-Liss, Inc.
Figures
Fig. 1
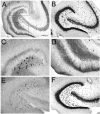
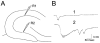
Differences in mossy fiber sprouting in the supra- and infrapyramidal blade of pilocarpine-treated rats with chronic seizures. A: A Timm-stained horizontal section of a pilocarpine-treated rat, killed 6 months after status epilepticus. A1: Timm-stained mossy fibers are distributed throughout the hilus (HIL), stratum lucidum, and the inner molecular layer. The suprapyramidal area, expanded in A2, shows less sprouting than the infrapyramidal blade, expanded in A3. MOL, molecular layer, GCL, granule cell layer. A2: The outlined section of the suprapyramidal blade shown in A1. A3: The outlined section of the infrapyramidal blade shown in A1. Note that sprouting is darker and more extensive in A3 than A2 (arrows). B: A BDNF–stained horizontal section of a different pilocarpine-treated rat than A. This animal was killed 4.5 months after pilocarpine-induced status epilepticus. B1: At low power, mossy fibers are visualized in the hilus, inner molecular layer, and area CA3. B2: The outlined section of the suprapyramidal blade shown in B1. B3: The outlined section of the infrapyramidal blade shown in B1. Note that sprouting is darker and more extensive in B3 relative to B2 (arrows). Scale bar in A1 = 200 μm in A1, 50 μm in A2,A3,B2,B3, 100 μm in B1.
Fig. 2
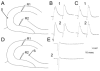
Blade asymmetry in mossy fiber sprouting equalize at the septal and temporal poles of the hippocampus. A: A coronal section through the septal hippocampus of a kainic acid-treated rat that was killed 2.5 months after status epilepticus and stained with antisera to neuropeptide Y (NPY) shows very little mossy fiber sprouting and homogeneous staining throughout the hilar mossy fibers. The sections shown in A–C were processed together. B: A horizontal section through the middle of the hippocampus of same animal as A. For A–C, the brain was rotated, after horizontal sections were cut, to examine the same hippocampus coronally at septal levels. This section, from the middle of the hippocampus, shows asymmetric sprouting (expanded in D,E). C: A horizontal section from the temporal pole of the same hippocampus as A,B. This section shows little asymmetry in sprouting. D,E: The sections outlined in B are expanded to allow better comparison of sprouting in the suprapyramidal and infrapyramidal blades. Scale bar in A = 300 μm in A–C, 75 μm in D,E.
Fig. 3
Preferential decrease in neuropeptide Y (NPY) immunoreactivity of sprouted mossy fibers at the lateral tip of the suprapyramidal blade in septal hippocampus of epileptic rats. A: A Timm-stained, coronal section from the septal hippocampus of a pilocarpine-treated rat that was killed 7 months after status epilepticus. There is relatively homogeneous sprouting in the suprapyramidal and infrapyramidal blades. MOL, molecular layer, GCL, granule cell layer; HIL, hilus. B: An adjacent section to the one in A, stained with antisera to BDNF, shows a similar degree of sprouting as in A. C: An adjacent section to the one in B, stained with antisera to NPY. Note the selective loss of NPY immunoreactivity in mossy fibers of the lateral tip of the suprapyramidal blade (arrows). Scale bar = 75 μm in A (applies to A–C).
Fig. 4
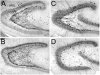
Asymmetry of neuropeptide Y (NPY) -immunoreactive mossy fibers and hilar neurons in epileptic rats. A: A NPY-stained section through the middle of the hippocampus of a pilocarpine-treated rat shows surviving hilar NPY-immunoreactive neurons are close to the suprapyramidal blade. This animal was killed 9 months after status epilepticus. B: An adjacent section to the one in A, stained with antisera to an antibody to a neuronal nuclear protein (NeuN), shows a considerable number of neurons located throughout the hilus. C,D: Areas of A are expanded in C (suprapyramidal) and D (infrapyramidal). Note the greater number of immunoreactive hilar neurons in C compared with D and the greater extent of sprouting in D relative to C. E: NPY staining in a horizontal section through the middle of the hippocampus in a saline-treated control rat that was killed 7 months after saline injection. Note the relatively homogeneous distribution of NPY-immunoreactive hilar cells in the control condition relative to the epileptic tissue shown in A. F: NeuN staining in an adjacent section to the one shown in E. Scale bar in A = 500 μm in A,B,E,F, 100 μm in C,D.
Fig. 5
Calretinin and parvalbumin immunoreactivity in pilocarpine-treated rats and saline-treated controls. A,B: Calretinin immunoreactivity in a pilocarpine-treated (A) and saline-treated (B) rat demonstrates preferential distribution of fibers in the suprapyramidal granule cell layer relative to the infrapyramidal blade (arrows). MOL, molecular layer, GCL, granule cell layer; HIL, hilus. C,D: Parvalbumin immunoreactivity in a pilocarpine-treated (C) and saline-treated (D) rat shows homogeneous immunoreactivity. Scale bar = 100 μm in A (applies to A–D).
Fig. 6
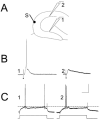
Extracellular recordings in slices from pilocarpine-treated rats with chronic seizures. A: A schematic diagram illustrates the recording arrangement for responses shown in B–D. The stimulating electrode was in the outer molecular layer at the crest (S). The stimulating electrode was monopolar and, thus, is diagrammed as a single line, with a small filled circle at the end to indicate where the electrode contacted the slice. The recording electrodes were equidistant from the stimulating electrode in the suprapyramidal (site R1) or infrapyramidal (site R2) blade. For this and all other dentate gyrus recordings, recording electrode depth was 50 μm; similar results were obtained at other depths. B: Responses to paired stimulation (identical stimuli triggered with a 20-msec interstimulus interval) of the outer molecular layer (as shown in A), recorded in the suprapyramidal (1) or infrapyramidal (2) blades. Stimuli evoked larger population spikes and less paired-pulse inhibition in the infrapyramidal blade. In this and other figures, the dots indicate the stimulus artifact. Stimulus artifacts were truncated. C: In a different slice, a single stimulus to the outer molecular layer (as shown in A) evoked one population spike in the suprapyramidal blade and two in the infrapyramidal blade. D: In a different slice, a pair of stimuli were triggered so that the population spike amplitudes evoked in response to the first stimulus were similar in amplitude; this was achieved by using a lower stimulus strength for the infrapyramidal blade recording than the suprapyramidal blade recording. Note that there was paired pulse inhibition at the suprapyramidal site but paired-pulse facilitation at the infrapyramidal site. E: A schematic diagram of the recording arrangement for the responses shown in F. The stimulating electrode was placed in the outer molecular layer in the center of the suprapyramidal blade (site S1) or the infrapyramidal blade (site S2). The recording site (R) was the pyramidal cell layer at the junction of area CA3b and CA3c. The depth of the recording was approximately 50 μm deep, where the maximal response was recorded in response to both stimuli. Similar results were obtained from other sites in area CA3b-c. F: The responses to two stimulation of site S1 and site S2 as shown in E. The response to stimulation of the suprapyramidal site (S1) was smaller than the response to the infrapyramidal stimulus (S2). Stimulus strength was identical. Stimuli were triggered 10 msec apart. The same result occurred regardless of the order of stimulation (i.e., site S1 was tested first or site S2 was first).
Fig. 7
Extracellular recordings of somatic and dendritic field potentials in response to molecular layer stimulation. A: A schematic of the recording and stimulation sites shows a single stimulus site in the outer molecular layer at the crest (S), and two pairs of recording sites: one in the suprapyramidal blade and the other in the infrapyramidal blade. For each pair of sites, one recording electrode was placed at the granule cell layer/hilar border (GCL) and the other site was positioned in the outer molecular layer at a location adjacent to the GCL electrode and at a depth that produced the maximal field excitatory postsynaptic potentials (MOL). B: Responses recorded simultaneously in the suprapyramidal blade GCL and MOL to a pair of identical crest stimuli, as shown in A. C: Responses of the infrapyramidal blade GCL and MOL recording sites to the same stimuli as those that were used to evoke the responses shown in B.
Fig. 8
Intracellular recordings from granule cells of the suprapyramidal and infrapyramidal blades of pilocarpine-treated rats with chronic seizures. A: A schematic of the recording and stimulating electrodes used to obtain the recordings shown in B. The stimulating electrode (S) was in the outer molecular layer at the crest. Intracellular recordings were made from granule cells of either the suprapyramidal blade (site R1) or the infrapyramidal blade (site R2). B: The response of two different granule cells that were sequentially impaled in the same slice. The same stimulus that triggered a single action potential in the suprapyramidal granule cell (1) evoked several action potentials in the infrapyramidal blade cell (2).
Fig. 9
Spontaneous epileptiform burst discharges after exposure of slices from pilocarpine-treated rats to BDNF. A: A schematic of the recording arrangement used to obtain the responses shown in B. There were two recording sites: one in the suprapyramidal blade (site R1) and the other in the infrapyramidal blade (site R2). B: Spontaneous epileptiform burst discharges recorded from the suprapyramidal (B1) and infrapyramidal (B2) blades of the same slice. BDNF (100 ng/ml) was added to the buffer that flowed over the slices (“bath-application”) 60 minutes before the recordings. The spontaneous events corresponded to large depolarizations and repetitive action potential discharge in granule cells (Scharfman et al., 1999). Note that spontaneous events were larger in amplitude and longer lasting in the infrapyramidal blade.
Fig. 10
Extracellular recordings from the suprapyramidal and infrapyramidal granule cell layer of normal adult rat hippocampal slices, in response to stimulation of the molecular layer or hilar/CA3 border. A: Schematic diagram of the recording arrangement for responses shown in B and C. The stimulation site was the outer molecular layer at the crest (S). Recording sites were equidistant from the stimulus site, either in the suprapyramidal (R1) or infrapyramidal blade (R2). B: Responses to two identical stimuli (20-msec interval) are shown, recorded at site 1 (B1) or site 2 (B2). Note that the same stimulus that evoked a small population spike in the suprapyramidal blade evoked a large population spike in the infrapyramidal blade. C: In a different slice from B, a similar recording and stimulating arrangement was tested and responses were similar in the two blades. Thus, blade asymmetry was not always present in normal rats. D: Schematic diagram of the recording arrangement for responses shown in E. The stimulus site was at the border of the hilus and the end of the CA3c pyramidal cell layer (S). Recording sites were similar to those in A. E: A fixed stimulus evoked similar antidromic population spikes in the two blades.
Fig. 11
Intracellular recordings of granule cells in the suprapyramidal and infrapyramidal blades of the same slice. A: Schematic diagram of the recording and stimulating arrangement used to record responses in B and C. The stimulating electrode (S) was placed at the outer molecular layer at the crest. Recording electrodes were equidistant from the stimulating electrode in the infrapyramidal blade (site 1) or suprapyramidal blade (site 2). B: Intracellularly recorded responses of an infrapyramidal cell at site 1 (left) and suprapyramidal cell at site 2 (right). The response to a 20-μsec, 100-μA stimulus evoked an action potential in the suprapyramidal cell but not the infrapyramidal cell. Even increasing the stimulus to 200 μsec, 100 μA did not evoke an action potential in the suprapyramidal cell (not shown). However, robust excitatory postsynaptic potentials were recorded in both cells. C: Responses of the same cells in B to intracellular injection of current. These responses show that threshold for action potential generation was a similar membrane potential in the two cells (a dotted line is provided to more easily compare threshold membrane potential). Intracellular current was injected as rectangular current pulses (150-msec duration). Calibration bar in C = 20 mV, 20 msec in B; 25 mV, 0.6 nA, 35 msec in C.
Fig. 12
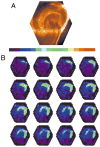
Asymmetry of fluorescent images in normal slices. A: A video image of a slice from which voltage imaging data (shown in B) was taken. This view shows the orientation and location of the slice as a reference for the fluorescence images in B. The microelectrode used for stimulation can be seen at the top of the image, in the molecular layer at the crest of the dentate gyrus. CA3 is down; CA1 is to the right. B: A time sequence of fluorescence images from the slice shown in A shows the spread of depolarization after the application of a 200-μsec, 100-μA stimulus to the crest in the middle molecular layer. Intensity was encoded in color according to the bar above, with higher fluorescence encoded as red and lower fluorescence encoded as blue. The images were taken at 0.7-msec intervals starting with the first frame after stimulation, with the sequence from left to right and top to bottom. The first row shows the initiation of a local depolarization as a small light green spot corresponding to the site of the stimulus pipette visible in A. The red signifies a large depolarization evoked by the stimulus, extending well into the infrapyramidal blade.
Fig. 13
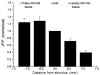
Quantification of fluorescent signals evoked by molecular layer stimulation. The peak value of ΔF/F was measured in response to a 200 μsec, 100 μA applied to the middle molecular layer at the crest. Responses are in the middle molecular layer at the indicated distances from the stimulus electrode. Responses were normalized to that in the crest, where the mean value of ΔF/F was 0.0023 ± 0.0004. Means with standard errors are shown for measurements in six slices.
Similar articles
- Recurrent excitatory connectivity in the dentate gyrus of kindled and kainic acid-treated rats.
Lynch M, Sutula T. Lynch M, et al. J Neurophysiol. 2000 Feb;83(2):693-704. doi: 10.1152/jn.2000.83.2.693. J Neurophysiol. 2000. PMID: 10669485 - Actions of brain-derived neurotrophic factor in slices from rats with spontaneous seizures and mossy fiber sprouting in the dentate gyrus.
Scharfman HE, Goodman JH, Sollas AL. Scharfman HE, et al. J Neurosci. 1999 Jul 1;19(13):5619-31. doi: 10.1523/JNEUROSCI.19-13-05619.1999. J Neurosci. 1999. PMID: 10377368 Free PMC article. - Recurrent mossy fiber pathway in rat dentate gyrus: synaptic currents evoked in presence and absence of seizure-induced growth.
Okazaki MM, Molnár P, Nadler JV. Okazaki MM, et al. J Neurophysiol. 1999 Apr;81(4):1645-60. doi: 10.1152/jn.1999.81.4.1645. J Neurophysiol. 1999. PMID: 10200201 - Neuropeptide Y in the dentate gyrus.
Sperk G, Hamilton T, Colmers WF. Sperk G, et al. Prog Brain Res. 2007;163:285-97. doi: 10.1016/S0079-6123(07)63017-9. Prog Brain Res. 2007. PMID: 17765725 Review. - Plasticity of neuropeptide Y in the dentate gyrus after seizures, and its relevance to seizure-induced neurogenesis.
Scharfman HE, Gray WP. Scharfman HE, et al. EXS. 2006;(95):193-211. doi: 10.1007/3-7643-7417-9_15. EXS. 2006. PMID: 16383008 Free PMC article. Review.
Cited by
- "Untangling" Alzheimer's disease and epilepsy.
Scharfman HE. Scharfman HE. Epilepsy Curr. 2012 Sep;12(5):178-83. doi: 10.5698/1535-7511-12.5.178. Epilepsy Curr. 2012. PMID: 23118602 Free PMC article. - An ancient duplication of exon 5 in the Snap25 gene is required for complex neuronal development/function.
Johansson JU, Ericsson J, Janson J, Beraki S, Stanić D, Mandic SA, Wikström MA, Hökfelt T, Ogren SO, Rozell B, Berggren PO, Bark C. Johansson JU, et al. PLoS Genet. 2008 Nov;4(11):e1000278. doi: 10.1371/journal.pgen.1000278. Epub 2008 Nov 28. PLoS Genet. 2008. PMID: 19043548 Free PMC article. - Grafting of striatal precursor cells into hippocampus shortly after status epilepticus restrains chronic temporal lobe epilepsy.
Hattiangady B, Rao MS, Shetty AK. Hattiangady B, et al. Exp Neurol. 2008 Aug;212(2):468-81. doi: 10.1016/j.expneurol.2008.04.040. Epub 2008 May 15. Exp Neurol. 2008. PMID: 18579133 Free PMC article. - Proximodistal segregation of nonspatial information in CA3: preferential recruitment of a proximal CA3-distal CA1 network in nonspatial recognition memory.
Nakamura NH, Flasbeck V, Maingret N, Kitsukawa T, Sauvage MM. Nakamura NH, et al. J Neurosci. 2013 Jul 10;33(28):11506-14. doi: 10.1523/JNEUROSCI.4480-12.2013. J Neurosci. 2013. PMID: 23843521 Free PMC article. - Disrupted hippocampal network physiology following PTEN deletion from newborn dentate granule cells.
LaSarge CL, Pun RY, Muntifering MB, Danzer SC. LaSarge CL, et al. Neurobiol Dis. 2016 Dec;96:105-114. doi: 10.1016/j.nbd.2016.09.004. Epub 2016 Sep 3. Neurobiol Dis. 2016. PMID: 27597527 Free PMC article.
References
- Alger BE, Teyler TJ. Long-term and short-term plasticity in the CA1, CA3, and dentate regions of the rat hippocampal slice. Brain Res. 1976;110:463–480. - PubMed
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182:851–914. - PubMed
- Amaral DG, Campbell MJ. Transmitter systems in the primate dentate gyrus. Hum Neurobiol. 1986;5:169–180. - PubMed
- Baimbridge KG. Calcium-binding proteins in the dentate gyrus. Epilepsy Res Suppl. 1992;7:211–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS38285/NS/NINDS NIH HHS/United States
- R56 NS037562/NS/NINDS NIH HHS/United States
- R01 NS037562/NS/NINDS NIH HHS/United States
- NS37212/NS/NINDS NIH HHS/United States
- NS37562/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous