Inhibition of SAPK2a/p38 prevents hnRNP A0 phosphorylation by MAPKAP-K2 and its interaction with cytokine mRNAs - PubMed (original) (raw)
Inhibition of SAPK2a/p38 prevents hnRNP A0 phosphorylation by MAPKAP-K2 and its interaction with cytokine mRNAs
Simon Rousseau et al. EMBO J. 2002.
Abstract
Lipopolysaccharide (LPS) stimulates production of inflammatory mediators, partly by stabilizing [interleukin-6 (IL-6), cyclooxygenase 2 (COX-2)] and/or stimulating translation [tumour necrosis factor-alpha (TNF-alpha)] of their mRNAs. Such regulation depends on AU-rich elements (AREs) within the 3'-untranslated regions and is partially suppressed by SB 203580 (which inhibits SAPK2a/p38). The LPS-induced production of TNF-alpha and IL-6 is suppressed in MAPKAP-K2-deficient mice (a kinase activated by SAPK2a/p38). Here, we identify 18 macrophage proteins that bind to AREs and show that hnRNP A0 is a major substrate for MAPKAP-K2 in this fraction. MAPKAP-K2 phosphorylated hnRNP A0 at Ser84 in vitro and this residue became phosphorylated in LPS-stimulated cells. Phosphorylation was prevented by SB 203580 and suppressed in macrophages derived from MAPKAP-K2-deficient mice. The mRNAs encoding TNF-alpha, COX-2 and macrophage inflammatory protein-2 (MIP-2) bound to hnRNP A0 in LPS-stimulated macrophages, an interaction prevented by SB 203580. The LPS-induced stabilization of MIP-2 mRNA and production of MIP-2 protein were abolished when macrophages were incubated with SB 203580 plus PD 184352 (which inhibits the classical MAP kinase cascade). Our data suggest that LPS-induced binding of hnRNP A0 to AREs may contribute to the post-transcriptional regulation of specific mRNAs.
Figures
Fig. 1. Identification of proteins that bind to the TNF-α ARE. Untreated RAW cell extracts were incubated with a biotinylated RNA oligonucleotide corresponding to the AU-rich 3′-UTRs of the TNF-α mRNA (UUAUUAUUUAUUAUUUAUUUAUUAUUUAUUUAUUU). The ARE-binding proteins were resolved on a two-dimensional gel covering the range from pH 6 to pH 11 and stained with Sypro-Ruby. Proteins were identified by tryptic mass fingerprinting (Table I).
Fig. 2. MAPKAP-K2 phosphorylates two major proteins binding to the TNF-α ARE. Untreated RAW cell extracts were incubated with a biotin ylated RNA oligonucleotide corresponding to the TNF-α ARE. RNA-binding proteins were then phosphorylated in the absence or presence of 1 or 10 U/ml of MAPKAP-K2 and 20 nM [γ-32P]ATP (lanes 1–4). Proteins were then separated by SDS–PAGE, transferred to nitrocellulose membranes and autoradiographed.
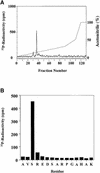
Fig. 3. Mapping of the site in hnRNP A0 phosphorylated by MAPKAP-K2. (A) Purified GST–hnRNP A0 was phosphorylated for 60 min in vitro with 150 U/ml of MAPKAP-K2 and 0.1 mM [γ-32P]ATP, and subjected to SDS–PAGE. The band corresponding to GST–hnRNP A0 was excised, digested with trypsin and the resulting peptides separated by reverse phase hydrophobic interaction chromatography on a C18 column equilibrated in 0.1% trifluoroacetic acid. 32P-radioactivity is shown by the full line, and the acetonitrile gradient by the broken line. (B) The mass of the major tryptic phosphopeptide from A (m/z = 1631.8) corresponds to that of a mono-phosphorylated form of a tryptic peptide comprising residues 82–96. The sequence of the peptide was confirmed by Edman sequencing and the site of phosphorylation was identified as the first serine in the peptide in a solid phase sequencing experiment. The methodology is detailed elsewhere (Stokoe et al., 1992).
Fig. 4. hnRNP A0 is phosphorylated at Ser84 in LPS-stimulated macrophages. (A) GST–hnRNP A0 was phosphorylated for 60 min in vitro with 10 U/ml of MAPKAP K2 (+) and 0.1 mM ATP or left unphos phorylated (–). The proteins were spotted onto a nitrocellulose membrane in the amounts indicated. Blots were probed with the phospho-specific antibody that recognizes hnRNP A0 phosphorylated at Ser84 in the presence of the phosphorylated peptide antigen (pSer84) or the unphosphorylated Ser84 peptide (both at 10 µg/ml). (B) RAW cells were pre-treated or not for 15 min with 10 µM SB 203580 (SB) and either left untreated or stimulated with 50 ng/ml LPS for the times indicated. Lysates were separated by SDS–PAGE and, after transfer to nitrocellulose, the membranes were probed with the phospho-specific Ser84 antibody. (C) BMDMs from wild-type and MAPKAP-K2-deficient mice were pre-treated or not for 15 min with 10 µM SB 203580 (SB) and either left untreated or stimulated with 50 ng/ml LPS for 1 h. The cells were lysed and hnRNP A0 immunoprecipitated and immunoblotted as described in (B). The upper panel was probed with the Ser84 phospho-specific antibody and the lower panel with an antibody that recognizes the phosphorylated and dephosphorylated forms of hnRNP A0 equally well.
Fig. 5. MIP-2 mRNA is bound by hnRNP A0 in extracts from LPS-stimulated cells. (A) RAW cells were left untreated or treated for 1 h with 50 ng/ml LPS and lysed. Then immunoprecipitation with (lanes 3–4) or without (lanes 1–2) the hnRNP A0 antibody was carried out from clarified extracts and the RNA bound identified using a differential display-based assay with random primer set 1 (see Materials and methods). CTL, control experiments. (B) Sequences of part of the 3′-UTR of murine MIP-2 mRNA (accession No. X53798) and TNF-α mRNA (accession No. X02611).
Fig. 6. SB 203580 prevents the LPS-induced interaction of hnRNP A0 with the mRNAs of MIP-2, COX-2 and TNF-α. RAW cells were pre-treated or not for 15 min with 10 µM SB 203580 (SB) or 1 µM PD 184352 (PD) and either left untreated or stimulated for 1 h with 50 ng/ml LPS. hnRNP A0 was immunoprecipitated as described in Figure 5 and mRNAs for MIP-2, TNF-α, COX-2 and IL-6 were amplified by RT–PCR with specific primers. CTL, control experiment.
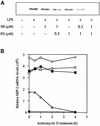
Fig. 7. The SAPK2a/p38 pathway and the classical MAP kinase cascade are both required for MIP-2 production at the mRNA and protein level. (A) RAW cells were pre-treated or not for 15 min with the indicated concentrations of SB 203580 (SB) and/or the indicated concentrations of PD 184352 (PD), and either left untreated or stimulated with 50 ng/ml LPS for 2 h. Lysates were separated by SDS–PAGE and, after transfer to nitrocellulose, the membranes were probed with a murine MIP-2-specific antibody. (B) RAW cells were treated for 15 min without (open and closed circles), with SB 203580 (closed squares), with PD 184352 (open squares) or SB 203580 plus PD 184352 (open triangles) and then for 1 h without (closed circles) or with (other symbols) LPS (time = 0 h). Transcription was then inhibited by the addition of actinomycin D and the level of MIP-2 RNA quantified at various times up to 4 h using an RNase protection assay.
Similar articles
- Participation of a stress-activated protein kinase cascade in the activation of tyrosine hydroxylase in chromaffin cells.
Thomas G, Haavik J, Cohen P. Thomas G, et al. Eur J Biochem. 1997 Aug 1;247(3):1180-9. doi: 10.1111/j.1432-1033.1997.01180.x. Eur J Biochem. 1997. PMID: 9288946 - Stress-induced regulation of eukaryotic elongation factor 2 kinase by SB 203580-sensitive and -insensitive pathways.
Knebel A, Haydon CE, Morrice N, Cohen P. Knebel A, et al. Biochem J. 2002 Oct 15;367(Pt 2):525-32. doi: 10.1042/BJ20020916. Biochem J. 2002. PMID: 12171600 Free PMC article. - Stress-induced phosphorylation of STAT1 at Ser727 requires p38 mitogen-activated protein kinase whereas IFN-gamma uses a different signaling pathway.
Kovarik P, Stoiber D, Eyers PA, Menghini R, Neininger A, Gaestel M, Cohen P, Decker T. Kovarik P, et al. Proc Natl Acad Sci U S A. 1999 Nov 23;96(24):13956-61. doi: 10.1073/pnas.96.24.13956. Proc Natl Acad Sci U S A. 1999. PMID: 10570180 Free PMC article. - MAPKAP kinases MK2 and MK3 in inflammation: complex regulation of TNF biosynthesis via expression and phosphorylation of tristetraprolin.
Ronkina N, Menon MB, Schwermann J, Tiedje C, Hitti E, Kotlyarov A, Gaestel M. Ronkina N, et al. Biochem Pharmacol. 2010 Dec 15;80(12):1915-20. doi: 10.1016/j.bcp.2010.06.021. Epub 2010 Jun 23. Biochem Pharmacol. 2010. PMID: 20599781 Review. - RNA-Binding Proteins in the Control of LPS-Induced Macrophage Response.
Ostareck DH, Ostareck-Lederer A. Ostareck DH, et al. Front Genet. 2019 Feb 4;10:31. doi: 10.3389/fgene.2019.00031. eCollection 2019. Front Genet. 2019. PMID: 30778370 Free PMC article. Review.
Cited by
- Post-transcriptional regulatory networks in immunity.
Ivanov P, Anderson P. Ivanov P, et al. Immunol Rev. 2013 May;253(1):253-72. doi: 10.1111/imr.12051. Immunol Rev. 2013. PMID: 23550651 Free PMC article. Review. - Heterogeneous nuclear ribonucleoprotein A/B: an emerging group of cancer biomarkers and therapeutic targets.
Lu Y, Wang X, Gu Q, Wang J, Sui Y, Wu J, Feng J. Lu Y, et al. Cell Death Discov. 2022 Jul 25;8(1):337. doi: 10.1038/s41420-022-01129-8. Cell Death Discov. 2022. PMID: 35879279 Free PMC article. Review. - The Role of Mitogen-Activated Protein Kinase-Activated Protein Kinases (MAPKAPKs) in Inflammation.
Moens U, Kostenko S, Sveinbjørnsson B. Moens U, et al. Genes (Basel). 2013 Mar 26;4(2):101-33. doi: 10.3390/genes4020101. Genes (Basel). 2013. PMID: 24705157 Free PMC article. - Organometallic Titanocene-Gold Compounds as Potential Chemotherapeutics in Renal Cancer. Study of their Protein Kinase Inhibitory Properties.
Fernández-Gallardo J, Elie BT, Sulzmaier FJ, Sanaú M, Ramos JW, Contel M. Fernández-Gallardo J, et al. Organometallics. 2014 Nov 24;33(22):6669-6681. doi: 10.1021/om500965k. Epub 2014 Oct 30. Organometallics. 2014. PMID: 25435644 Free PMC article. - hnRNP A/B Proteins: An Encyclopedic Assessment of Their Roles in Homeostasis and Disease.
Thibault PA, Ganesan A, Kalyaanamoorthy S, Clarke JWE, Salapa HE, Levin MC. Thibault PA, et al. Biology (Basel). 2021 Jul 24;10(8):712. doi: 10.3390/biology10080712. Biology (Basel). 2021. PMID: 34439945 Free PMC article. Review.
References
- Caivano M. and Cohen,P. (2000) Role of mitogen-activated protein kinase cascades in mediating lipopolysaccharide-stimulated induction of cyclooxygenase-2 and IL-1β in RAW264 macrophages. J. Immunol., 164, 3018–3025. - PubMed
- Dumitru C.D. et al. (2000) TNF-α induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell, 103, 1071–1083. - PubMed
- Eyers P.A., van den,I.P., Quinlan,R.A., Goedert,M. and Cohen,P. (1999) Use of a drug-resistant mutant of stress-activated protein kinase 2a/p38 to validate the in vivo specificity of SB 203580. FEBS Lett., 451, 191–196. - PubMed
- Grzybowska E.A., Wilczynska,A. and Siedlecki,J.A. (2001) Regulatory functions of 3′UTRs. Biochem. Biophys. Res. Commun., 288, 291–295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous