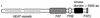Elucidating TOR signaling and rapamycin action: lessons from Saccharomyces cerevisiae - PubMed (original) (raw)
Review
Elucidating TOR signaling and rapamycin action: lessons from Saccharomyces cerevisiae
José L Crespo et al. Microbiol Mol Biol Rev. 2002 Dec.
Abstract
TOR (target of rapamycin) is a phosphatidylinositol kinase-related protein kinase that controls cell growth in response to nutrients. Rapamycin is an immunosuppressive and anticancer drug that acts by inhibiting TOR. The modes of action of TOR and rapamycin are remarkably conserved from S. cerevisiae to humans. The current understanding of TOR and rapamycin is derived largely from studies with S. cerevisiae. In this review, we discuss the contributions made by S. cerevisiae to understanding rapamycin action and TOR function.
Figures
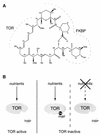
FIG. 1.
Rapamycin-FKBP complex binds and inhibits TOR. (A) Chemical structure of rapamycin. The TOR- and FKBP-interacting regions of rapamycin (30) are indicated by dashed lines. (B) TOR is active in the presence of nutrients and inactive upon nutrient limitation or FKBP-rapamycin (Rap) binding.
FIG. 2.
Structure of the TOR kinases. Functional domains conserved in TOR proteins are depicted, including the N-terminal HEAT repeats, the central FAT domain, and the C-terminal FKBP-rapamycin binding (FRB), kinase, and FATC domains. aa, amino acids.
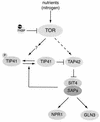
FIG. 3.
TOR controls phosphatases in S. cerevisiae. Under good nutrient (nitrogen) conditions, TOR inhibits the phosphatase SIT4 by promoting the association of SIT4 with TAP42. Two different models have been proposed for the mechanism by which TOR controls the SIT4-TAP42 complex. Jiang and Broach (81) proposed that TOR controls the interaction between SIT4 and TAP42 by phosphorylating TAP42 directly (indicated by a dashed arrow). Jacinto et al. (79) suggested that the association of SIT4 and TAP42 is controlled primarily by the TAP42 interactor TIP41. TOR may phosphorylate and inactivate TIP41 by an unknown mechanism. Dephosphorylated TIP41 positively regulates SIT4 by binding and inhibiting TAP42. The association of TIP41 with TAP42 enhances SIT4 phosphatase activity, allowing free SIT4 subunits to associate with SAPs and activate target phosphoproteins such as NPR1 and GLN3. Dephosphorylation and activation of TIP41 are mediated by SIT4, indicating that TIP41 is part of a feedback loop that amplifies SIT4 activity. Arrows indicate activation; bars indicate inhibition. Adapted from Jacinto et al. (79). Rap, rapamycin.
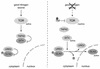
FIG. 4.
TOR inhibits autophagy in S. cerevisiae. Under good nutrient conditions, TOR inhibits autophagy by promoting phosphorylation (P) of APG13 and thereby preventing the formation of an APG1-APG13 complex, which is essential for the induction of autophagy. Inactivation of TOR by rapamycin (Rap) treatment or nutrient deprivation results in rapid dephosphorylation of APG13. Dephosphorylated APG13 associates with APG1, and the active APG13-APG1 complex induces autophagy. Arrows indicate activation; bars indicate inhibition.
FIG. 5.
TOR prevents nuclear accumulation of the nitrogen-regulated transcription activator GLN3 via TAP42-mediated inhibition of the phosphatase SIT4. Under good nitrogen conditions, GLN3 is phosphorylated and retained in the cytoplasm by URE2. Upon nitrogen starvation or rapamycin treatment, SIT4 is released from TAP42 and activated. Activated SIT4 dephosphorylates the GATA transcription factor GLN3. Dephosphorylated GLN3 dissociates from URE2 and translocates into the nucleus, where it activates transcription of target genes. Arrows indicate activation; bars indicate inhibition.
Similar articles
- Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control.
Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Loewith R, et al. Mol Cell. 2002 Sep;10(3):457-68. doi: 10.1016/s1097-2765(02)00636-6. Mol Cell. 2002. PMID: 12408816 - The fission yeast TOR proteins and the rapamycin response: an unexpected tale.
Weisman R. Weisman R. Curr Top Microbiol Immunol. 2004;279:85-95. doi: 10.1007/978-3-642-18930-2_6. Curr Top Microbiol Immunol. 2004. PMID: 14560953 Review. - Nutrient signaling through TOR kinases controls gene expression and cellular differentiation in fungi.
Rohde JR, Cardenas ME. Rohde JR, et al. Curr Top Microbiol Immunol. 2004;279:53-72. doi: 10.1007/978-3-642-18930-2_4. Curr Top Microbiol Immunol. 2004. PMID: 14560951 Review. - Rapamycin and less immunosuppressive analogs are toxic to Candida albicans and Cryptococcus neoformans via FKBP12-dependent inhibition of TOR.
Cruz MC, Goldstein AL, Blankenship J, Del Poeta M, Perfect JR, McCusker JH, Bennani YL, Cardenas ME, Heitman J. Cruz MC, et al. Antimicrob Agents Chemother. 2001 Nov;45(11):3162-70. doi: 10.1128/AAC.45.11.3162-3170.2001. Antimicrob Agents Chemother. 2001. PMID: 11600372 Free PMC article. - The fission yeast TOR homolog, tor1+, is required for the response to starvation and other stresses via a conserved serine.
Weisman R, Choder M. Weisman R, et al. J Biol Chem. 2001 Mar 9;276(10):7027-32. doi: 10.1074/jbc.M010446200. Epub 2000 Nov 28. J Biol Chem. 2001. PMID: 11096119
Cited by
- Toward the discovery of biological functions associated with the mechanosensor Mtl1p of Saccharomyces cerevisiae via integrative multi-OMICs analysis.
Martínez-Matías N, Chorna N, González-Crespo S, Villanueva L, Montes-Rodríguez I, Melendez-Aponte LM, Roche-Lima A, Carrasquillo-Carrión K, Santiago-Cartagena E, Rymond BC, Babu M, Stagljar I, Rodríguez-Medina JR. Martínez-Matías N, et al. Sci Rep. 2021 Apr 1;11(1):7411. doi: 10.1038/s41598-021-86671-8. Sci Rep. 2021. PMID: 33795741 Free PMC article. - INK128 Exhibits Synergy with Azoles against Exophiala spp. and Fusarium spp.
Gao L, Sun Y, He C, Li M, Zeng T, Lu Q. Gao L, et al. Front Microbiol. 2016 Oct 20;7:1658. doi: 10.3389/fmicb.2016.01658. eCollection 2016. Front Microbiol. 2016. PMID: 27812353 Free PMC article. - Signaling by target of rapamycin proteins in cell growth control.
Inoki K, Ouyang H, Li Y, Guan KL. Inoki K, et al. Microbiol Mol Biol Rev. 2005 Mar;69(1):79-100. doi: 10.1128/MMBR.69.1.79-100.2005. Microbiol Mol Biol Rev. 2005. PMID: 15755954 Free PMC article. Review. - TOR regulates the subcellular localization of Ime1, a transcriptional activator of meiotic development in budding yeast.
Colomina N, Liu Y, Aldea M, Garí E. Colomina N, et al. Mol Cell Biol. 2003 Oct;23(20):7415-24. doi: 10.1128/MCB.23.20.7415-7424.2003. Mol Cell Biol. 2003. PMID: 14517308 Free PMC article. - Proteaphagy is specifically regulated and requires factors dispensable for general autophagy.
Waite KA, Burris A, Vontz G, Lang A, Roelofs J. Waite KA, et al. J Biol Chem. 2022 Jan;298(1):101494. doi: 10.1016/j.jbc.2021.101494. Epub 2021 Dec 14. J Biol Chem. 2022. PMID: 34919962 Free PMC article.
References
- Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. - PubMed
- Andrade, M. A., and P. Bork. 1995. HEAT repeats in the Huntington's disease protein. Nat. Genet. 11:115-116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases