Biliverdin reductase: a major physiologic cytoprotectant - PubMed (original) (raw)
Biliverdin reductase: a major physiologic cytoprotectant
David E Baranano et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Bilirubin, an abundant pigment that causes jaundice, has long lacked any clear physiologic role. It arises from enzymatic reduction by biliverdin reductase of biliverdin, a product of heme oxygenase activity. Bilirubin is a potent antioxidant that we show can protect cells from a 10,000-fold excess of H2O2. We report that bilirubin is a major physiologic antioxidant cytoprotectant. Thus, cellular depletion of bilirubin by RNA interference markedly augments tissue levels of reactive oxygen species and causes apoptotic cell death. Depletion of glutathione, generally regarded as a physiologic antioxidant cytoprotectant, elicits lesser increases in reactive oxygen species and cell death. The potent physiologic antioxidant actions of bilirubin reflect an amplification cycle whereby bilirubin, acting as an antioxidant, is itself oxidized to biliverdin and then recycled by biliverdin reductase back to bilirubin. This redox cycle may constitute the principal physiologic function of bilirubin.
Figures
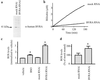
Fig 1.
Bilirubin is a suprastoichiometric cytoprotectant against oxidative stress (a) and BVRA can redox cycle the tetrapyrrole in vitro (b_–_d). (a) Viability of cultured HeLa cells was measured by MTT assay 8 h after the indicated treatment. (b) Bilirubin (Porphyrin Products) is oxidized to biliverdin by peroxyl radicals. The reaction was monitored by _A_453 for bilirubin (□) or _A_650 for biliverdin (•). (c) Bilirubin IXα is oxidized specifically to biliverdin IXα. An aliquot of the reaction shown in b was taken at 10 min and analyzed by HPLC as described (14). (d) BVRA regenerates bilirubin, slowing the consumption of bilirubin by ROS. The reaction was monitored at _A_453 with (□) or without (•) 1 μg/ml recombinant BVRA. *, P < 0.005, using an unpaired Student's t test.
Fig 2.
Depletion of BVRA with RNAi leads to elevated ROS levels in mammalian cells. (a) Expression of the BVRA protein is decreased following transfection with RNAi directed to the BVRA transcript. (b) Biliverdin reductase activity is decreased following treatment with RNAi directed to the BVRA transcript. (c) Intracellular ROS levels are higher in HeLa cells deficient in BVRA. ROS levels were measured using H2DCF. (d) Intracellular ROS levels are higher in neurons transfected with RNAi directed to the BVRA transcript. *, P < 0.005 using an unpaired Student's t test.
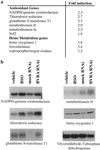
Fig 3.
(a) Microarray analysis of BVRA-deficient cells indicates increased expression of antioxidant genes. Heme synthesis as well as degradation is up-regulated. Of the 12,599 genes represented, 4,368 genes were detected. Of these genes, 645 were significantly increased in the BVRA-deficient condition and 550 were significantly decreased. Many antioxidant genes were increased in the cells deficient in BVRA. The average fold increase in the normalized signal is indicated. (b) To confirm the microarray data, Northern blot analysis was performed for some of the genes, comparing the induction to that of cells depleted of GSH by BSO treatment. HO1 expression was analyzed by Western blot.
Fig 4.
Depletion of BVRA leads to apoptosis and increased sensitivity to oxidative stress. (a) Cells depleted of BVRA are less viable 50 h after transfection. (b) Caspase activity is elevated in cells treated with BVRA RNAi (○) compared with two distinct mock RNAis (▾ and ▴). (c) Cell death is apoptotic, as indicated by poly(ADP-ribose) polymerase (PARP) cleavage, 50 h after transfection. (d) Cells deficient in BVRA (○) are more sensitive to an oxidative challenge than mock RNAi-treated cells (▾ and ▴). Thirty hours after transfection, the cells were treated with H2O2 and cell survival was measured. (e) In contrast, GSH depletion with BSO (○) treatment leads to only a small increase in sensitivity, significant only at 200 μM H2O2. *, P < 0.005 using an unpaired Student's t test.
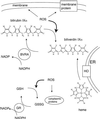
Fig 5.
A model for cytoprotection conferred by BVR. HO control the total level of biliverdin/bilirubin. BVRA keeps the linear tetrapyrrole in the reduced form, where it associates with membranes and quenches the propagation of ROS. This confers protection to the membrane components of the cell in much the same way as soluble GSH and GSH reductase (GR) protect the cytoplasmic components. M, methyl; V, vinyl; P, propionate; ER, endoplasmic reticulum; GSH, GSH-reduced; GSSG, GSH-oxidized.
Comment in
- The jaundice of the cell.
Greenberg DA. Greenberg DA. Proc Natl Acad Sci U S A. 2002 Dec 10;99(25):15837-9. doi: 10.1073/pnas.012685199. Epub 2002 Dec 2. Proc Natl Acad Sci U S A. 2002. PMID: 12461187 Free PMC article. No abstract available.
Similar articles
- Bilirubin and glutathione have complementary antioxidant and cytoprotective roles.
Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, Snyder SH. Sedlak TW, et al. Proc Natl Acad Sci U S A. 2009 Mar 31;106(13):5171-6. doi: 10.1073/pnas.0813132106. Epub 2009 Mar 13. Proc Natl Acad Sci U S A. 2009. PMID: 19286972 Free PMC article. - Limited role for the bilirubin-biliverdin redox amplification cycle in the cellular antioxidant protection by biliverdin reductase.
Maghzal GJ, Leck MC, Collinson E, Li C, Stocker R. Maghzal GJ, et al. J Biol Chem. 2009 Oct 23;284(43):29251-9. doi: 10.1074/jbc.M109.037119. Epub 2009 Aug 18. J Biol Chem. 2009. PMID: 19690164 Free PMC article. - Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin.
Jansen T, Hortmann M, Oelze M, Opitz B, Steven S, Schell R, Knorr M, Karbach S, Schuhmacher S, Wenzel P, Münzel T, Daiber A. Jansen T, et al. J Mol Cell Cardiol. 2010 Aug;49(2):186-95. doi: 10.1016/j.yjmcc.2010.04.011. Epub 2010 Apr 27. J Mol Cell Cardiol. 2010. PMID: 20430037 - Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle.
Sedlak TW, Snyder SH. Sedlak TW, et al. Pediatrics. 2004 Jun;113(6):1776-82. doi: 10.1542/peds.113.6.1776. Pediatrics. 2004. PMID: 15173506 Review. No abstract available. - Antioxidant activities of bile pigments.
Stocker R. Stocker R. Antioxid Redox Signal. 2004 Oct;6(5):841-9. doi: 10.1089/ars.2004.6.841. Antioxid Redox Signal. 2004. PMID: 15345144 Review.
Cited by
- Teaching an old drug new tricks: Regulatory insights for the repurposing of hemin in cardiovascular disease.
Eadie AL, Simpson JA, Brunt KR. Eadie AL, et al. Pharmacol Res Perspect. 2024 Aug;12(4):e1225. doi: 10.1002/prp2.1225. Pharmacol Res Perspect. 2024. PMID: 38923404 Free PMC article. Review. - Antioxidant roles of heme oxygenase, carbon monoxide, and bilirubin in cerebral circulation during seizures.
Parfenova H, Leffler CW, Basuroy S, Liu J, Fedinec AL. Parfenova H, et al. J Cereb Blood Flow Metab. 2012 Jun;32(6):1024-34. doi: 10.1038/jcbfm.2012.13. Epub 2012 Feb 22. J Cereb Blood Flow Metab. 2012. PMID: 22354150 Free PMC article. - Serum bilirubin and the risk of hypertension.
Wang L, Bautista LE. Wang L, et al. Int J Epidemiol. 2015 Feb;44(1):142-52. doi: 10.1093/ije/dyu242. Epub 2014 Dec 25. Int J Epidemiol. 2015. PMID: 25541554 Free PMC article. - Risk factors for the time to development of retinopathy of prematurity in premature infants in Iran: a machine learning approach.
Tapak L, Farahani LN, Taleghani NT, Ebrahimiadib N, Pour EK, Farahani AD, Hamidi O. Tapak L, et al. BMC Ophthalmol. 2024 Aug 23;24(1):364. doi: 10.1186/s12886-024-03637-w. BMC Ophthalmol. 2024. PMID: 39180010 Free PMC article. - CD163 and inflammation: biological, diagnostic, and therapeutic aspects.
Etzerodt A, Moestrup SK. Etzerodt A, et al. Antioxid Redox Signal. 2013 Jun 10;18(17):2352-63. doi: 10.1089/ars.2012.4834. Epub 2012 Oct 19. Antioxid Redox Signal. 2013. PMID: 22900885 Free PMC article. Review.
References
- Orth J. (1875) Virchows Arch. Pathol. Anat. 63, 447-462.
- Schmid R. (1976) Trans. Assoc. Am. Physicians 89, 64-76. - PubMed
- Yamaguchi T. & Nakajima, H. (1995) Eur. J. Biochem. 233, 467-472. - PubMed
- Yamaguchi T., Komoda, Y. & Nakajima, H. (1994) J. Biol. Chem. 269, 24343-24348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA-00266/DA/NIDA NIH HHS/United States
- F30 DA005900/DA/NIDA NIH HHS/United States
- DA-00074/DA/NIDA NIH HHS/United States
- P50 DA000266/DA/NIDA NIH HHS/United States
- DA05900/DA/NIDA NIH HHS/United States
- K05 DA000074/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous