Essential role for the C5a receptor in regulating the effector phase of synovial infiltration and joint destruction in experimental arthritis - PubMed (original) (raw)
Essential role for the C5a receptor in regulating the effector phase of synovial infiltration and joint destruction in experimental arthritis
Ethan P Grant et al. J Exp Med. 2002.
Abstract
A characteristic feature of rheumatoid arthritis is the abundance of inflammatory cells in the diseased joint. Two major components of this infiltrate are neutrophils in the synovial fluid and macrophages in the synovial tissue. These cells produce cytokines including tumor necrosis factor alpha and other proinflammatory mediators that likely drive the disease through its effector phases. To investigate what mechanisms underlie the recruitment of these cells into the synovial fluid and tissue, we performed expression analyses of chemoattractant receptors in a related family that includes the anaphylatoxin receptors and the formyl-MetLeuPhe receptor. We then examined the effect of targeted disruption of two abundantly expressed chemoattractant receptors, the receptors for C3a and C5a, on arthritogenesis in a mouse model of disease. We report that genetic ablation of C5a receptor expression completely protects mice from arthritis.
Figures
Figure 1.
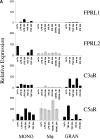
Expression of C5aR in human RA tissue and in a mouse model of arthritis. (A) Quantitative PCR analysis of C5aR, C3aR, FPRL1, and FPRL2 in untreated (UnTx) or activated (LPS, TNFα, IFNγ, or CD40L for 4 or 24 h) normal human peripheral blood monocytes (MONO), monocyte-derived macrophages (Mφ), and peripheral blood–derived granulocytes (GRAN). Data plotted are the mean ± SD of the relative expression of each receptor in RNA samples pooled from three different donors using β2-microglobulin as a reference. The results are representative of three independent experiments. (B) Immunohistochemistry analysis of human RA synovial tissue stained with anti-C5aR, anti-C3aR, anti-CD68, or control IgG. Data shown are representative of expression patterns observed of four RA synovial tissues. (C) Quantitative PCR analysis of C5aR, C3aR, and inflammatory marker expression in mouse joint RNA during the course of arthritis induction. Data are the mean ± SD of the relative expression of each gene in two joints.
Figure 1.
Expression of C5aR in human RA tissue and in a mouse model of arthritis. (A) Quantitative PCR analysis of C5aR, C3aR, FPRL1, and FPRL2 in untreated (UnTx) or activated (LPS, TNFα, IFNγ, or CD40L for 4 or 24 h) normal human peripheral blood monocytes (MONO), monocyte-derived macrophages (Mφ), and peripheral blood–derived granulocytes (GRAN). Data plotted are the mean ± SD of the relative expression of each receptor in RNA samples pooled from three different donors using β2-microglobulin as a reference. The results are representative of three independent experiments. (B) Immunohistochemistry analysis of human RA synovial tissue stained with anti-C5aR, anti-C3aR, anti-CD68, or control IgG. Data shown are representative of expression patterns observed of four RA synovial tissues. (C) Quantitative PCR analysis of C5aR, C3aR, and inflammatory marker expression in mouse joint RNA during the course of arthritis induction. Data are the mean ± SD of the relative expression of each gene in two joints.
Figure 1.
Expression of C5aR in human RA tissue and in a mouse model of arthritis. (A) Quantitative PCR analysis of C5aR, C3aR, FPRL1, and FPRL2 in untreated (UnTx) or activated (LPS, TNFα, IFNγ, or CD40L for 4 or 24 h) normal human peripheral blood monocytes (MONO), monocyte-derived macrophages (Mφ), and peripheral blood–derived granulocytes (GRAN). Data plotted are the mean ± SD of the relative expression of each receptor in RNA samples pooled from three different donors using β2-microglobulin as a reference. The results are representative of three independent experiments. (B) Immunohistochemistry analysis of human RA synovial tissue stained with anti-C5aR, anti-C3aR, anti-CD68, or control IgG. Data shown are representative of expression patterns observed of four RA synovial tissues. (C) Quantitative PCR analysis of C5aR, C3aR, and inflammatory marker expression in mouse joint RNA during the course of arthritis induction. Data are the mean ± SD of the relative expression of each gene in two joints.
Figure 2.
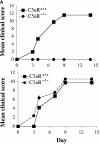
C5aR−/− mice are protected from arthritis induction. (A) Mean clinical scores at days 0, 3, 4, 7, 9, and 14 of arthritis development in C3aR−/− (bottom) or C5aR−/− (top) mice and their littermate controls. Each group of mice consisted of five animals. The results shown are representative of three separate experiments. (B) H&E stained joint sections from representative C3aR−/−, C3aR+/+, C5aR−/−, and C5aR+/+ mice 14 d after mAb transfer. Cartilage (C) surfaces are indicated in the images as are regions of pannus tissue (P) comprised of proliferating synoviocytes and infiltrating leukocytes. (C) Joints from naive C5aR+/+ mice. Ab-injected C5aR+/+ or Ab-injected C5aR−/− mice were stained with an antibody against mouse IgG to detect deposition (brown staining, top). Inflamed joints from C5aR+/+ mice 14 d after Ab transfer were stained with control IgG, anti-C3, or anti-C1q antibodies to detect the accumulation of complement components on the cartilage surfaces (bottom).
Figure 2.
C5aR−/− mice are protected from arthritis induction. (A) Mean clinical scores at days 0, 3, 4, 7, 9, and 14 of arthritis development in C3aR−/− (bottom) or C5aR−/− (top) mice and their littermate controls. Each group of mice consisted of five animals. The results shown are representative of three separate experiments. (B) H&E stained joint sections from representative C3aR−/−, C3aR+/+, C5aR−/−, and C5aR+/+ mice 14 d after mAb transfer. Cartilage (C) surfaces are indicated in the images as are regions of pannus tissue (P) comprised of proliferating synoviocytes and infiltrating leukocytes. (C) Joints from naive C5aR+/+ mice. Ab-injected C5aR+/+ or Ab-injected C5aR−/− mice were stained with an antibody against mouse IgG to detect deposition (brown staining, top). Inflamed joints from C5aR+/+ mice 14 d after Ab transfer were stained with control IgG, anti-C3, or anti-C1q antibodies to detect the accumulation of complement components on the cartilage surfaces (bottom).
Figure 2.
C5aR−/− mice are protected from arthritis induction. (A) Mean clinical scores at days 0, 3, 4, 7, 9, and 14 of arthritis development in C3aR−/− (bottom) or C5aR−/− (top) mice and their littermate controls. Each group of mice consisted of five animals. The results shown are representative of three separate experiments. (B) H&E stained joint sections from representative C3aR−/−, C3aR+/+, C5aR−/−, and C5aR+/+ mice 14 d after mAb transfer. Cartilage (C) surfaces are indicated in the images as are regions of pannus tissue (P) comprised of proliferating synoviocytes and infiltrating leukocytes. (C) Joints from naive C5aR+/+ mice. Ab-injected C5aR+/+ or Ab-injected C5aR−/− mice were stained with an antibody against mouse IgG to detect deposition (brown staining, top). Inflamed joints from C5aR+/+ mice 14 d after Ab transfer were stained with control IgG, anti-C3, or anti-C1q antibodies to detect the accumulation of complement components on the cartilage surfaces (bottom).
Figure 3.
Quantitative assessment of inflammatory markers in C5aR+/+ and C5aR−/− mouse joints. RNA was extracted from the joints of naive wild-type or mAb-injected C5aR+/+ (+/+ A) or mAb-injected C5aR−/− (−/− A) mice 13 d after mAb transfer and analyzed via quantitative PCR analysis for the indicated genes. The levels of IL-1β, CD68, TNFα, CD4, MMP-3, and OPGL were significantly reduced in the joints from C5aR−/− mice injected with the arthritogenic antibodies (−/− A) compared with injected C5aR+/+ mice (+/+ A; P < 0.0001, Student's t test). Data are the mean ± SD of the relative expression of each gene in ten joints each from naive mice or from mAb-injected C5aR−/− or C5aR+/+ mice. The results are representative of three independent experiments.
Figure 4.
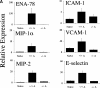
Decrease in neutrophilia, chemokines, and adhesion receptor induction in C5aR−/− mice. (A) Neutrophil chemotactic factors ENA-78 (P < 0.0001, Student's t test), MIP-1α (P < 0.0001), and MIP-2 (P < 0.0001), and adhesion molecules ICAM-1 (P < 0.002), VCAM-1 (P < 0.0001), and E-selectin (P < 0.0001) were significantly reduced in joints from C5aR−/− mice injected with the arthritogenic antibodies (−/− A) compared with injected C5aR+/+ mice (+/+ A). Quantitative PCR data are expressed as described in Fig. 3. (B) Neutrophilia is reduced in joints from C5aR−/− mice injected with the arthritogenic antibodies compared with injected C5aR+/+ mice as assessed by an assay for MPO performed with protein extracts from five joints each from C5aR+/+ and C5aR−/− mice 14 d after mAb transfer (P < 0.005). (C) Neutrophilia (N) in arthritic C5aR+/+ joints (top) is evident from examination of high powered fields of tissue sections stained with H&E. In contrast, only rare neutrophils were observed in joints from C5aR−/− animals 14 d after mAb transfer. Arrowheads indicate region of lower power fields (left) enlarged in the high power fields (right). Cartilage (C), pannus (P), and synovial lining (S) are indicated.
Figure 4.
Decrease in neutrophilia, chemokines, and adhesion receptor induction in C5aR−/− mice. (A) Neutrophil chemotactic factors ENA-78 (P < 0.0001, Student's t test), MIP-1α (P < 0.0001), and MIP-2 (P < 0.0001), and adhesion molecules ICAM-1 (P < 0.002), VCAM-1 (P < 0.0001), and E-selectin (P < 0.0001) were significantly reduced in joints from C5aR−/− mice injected with the arthritogenic antibodies (−/− A) compared with injected C5aR+/+ mice (+/+ A). Quantitative PCR data are expressed as described in Fig. 3. (B) Neutrophilia is reduced in joints from C5aR−/− mice injected with the arthritogenic antibodies compared with injected C5aR+/+ mice as assessed by an assay for MPO performed with protein extracts from five joints each from C5aR+/+ and C5aR−/− mice 14 d after mAb transfer (P < 0.005). (C) Neutrophilia (N) in arthritic C5aR+/+ joints (top) is evident from examination of high powered fields of tissue sections stained with H&E. In contrast, only rare neutrophils were observed in joints from C5aR−/− animals 14 d after mAb transfer. Arrowheads indicate region of lower power fields (left) enlarged in the high power fields (right). Cartilage (C), pannus (P), and synovial lining (S) are indicated.
Figure 4.
Decrease in neutrophilia, chemokines, and adhesion receptor induction in C5aR−/− mice. (A) Neutrophil chemotactic factors ENA-78 (P < 0.0001, Student's t test), MIP-1α (P < 0.0001), and MIP-2 (P < 0.0001), and adhesion molecules ICAM-1 (P < 0.002), VCAM-1 (P < 0.0001), and E-selectin (P < 0.0001) were significantly reduced in joints from C5aR−/− mice injected with the arthritogenic antibodies (−/− A) compared with injected C5aR+/+ mice (+/+ A). Quantitative PCR data are expressed as described in Fig. 3. (B) Neutrophilia is reduced in joints from C5aR−/− mice injected with the arthritogenic antibodies compared with injected C5aR+/+ mice as assessed by an assay for MPO performed with protein extracts from five joints each from C5aR+/+ and C5aR−/− mice 14 d after mAb transfer (P < 0.005). (C) Neutrophilia (N) in arthritic C5aR+/+ joints (top) is evident from examination of high powered fields of tissue sections stained with H&E. In contrast, only rare neutrophils were observed in joints from C5aR−/− animals 14 d after mAb transfer. Arrowheads indicate region of lower power fields (left) enlarged in the high power fields (right). Cartilage (C), pannus (P), and synovial lining (S) are indicated.
Figure 5.
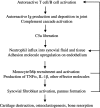
Model for the inflammatory cascade of arthritis. After the initiation of disease as a result of the activation of autoreactive T and B cells, rheumatoid factor and other autoantibodies are produced and deposited in the joint spaces. Immune complex formation leads to the activation of the complement cascade, ultimately yielding C5a. The C5a gradient emanating from the joint recruits and activates neutrophils and monocytes to the synovium and synovial fluid. In addition, C5a may directly activate endothelial cells to up-regulate adhesion receptor expression, promoting extravasation of inflammatory cells. Activated neutrophils liberate additional chemotactic factors that recruit additional inflammatory cell subsets, particularly monocyte/macrophage lineage cells. These latter cells, a primary source of TNFα and IL-1β, activate resident synovial fibroblasts. Activated synovial fibroblasts produce additional effector molecules, including cytokines, chemokines, and proteases. The end result is a cycle of positive feedback that amplifies the inflammation and results in cartilage and bone degradation.
Similar articles
- Comparison of the suppressive effects of soluble CR1 and C5a receptor antagonist in acute arthritis induced in rats by blocking of CD59.
Mizuno M, Nishikawa K, Morgan BP, Matsuo S. Mizuno M, et al. Clin Exp Immunol. 2000 Feb;119(2):368-75. doi: 10.1046/j.1365-2249.2000.01127.x. Clin Exp Immunol. 2000. PMID: 10632677 Free PMC article. - Role of C3a receptors, C5a receptors, and complement protein C6 deficiency in collagen antibody-induced arthritis in mice.
Banda NK, Hyatt S, Antonioli AH, White JT, Glogowska M, Takahashi K, Merkel TJ, Stahl GL, Mueller-Ortiz S, Wetsel R, Arend WP, Holers VM. Banda NK, et al. J Immunol. 2012 Feb 1;188(3):1469-78. doi: 10.4049/jimmunol.1102310. Epub 2011 Dec 28. J Immunol. 2012. PMID: 22205026 Free PMC article. - Disruption of the C5a receptor gene fails to protect against experimental allergic encephalomyelitis.
Reiman R, Gerard C, Campbell IL, Barnum SR. Reiman R, et al. Eur J Immunol. 2002 Apr;32(4):1157-63. doi: 10.1002/1521-4141(200204)32:4<1157::AID-IMMU1157>3.0.CO;2-M. Eur J Immunol. 2002. PMID: 11932923 - The critical role of C5a as an initiator of neutrophil-mediated autoimmune inflammation of the joint and skin.
Sadik CD, Miyabe Y, Sezin T, Luster AD. Sadik CD, et al. Semin Immunol. 2018 Jun;37:21-29. doi: 10.1016/j.smim.2018.03.002. Epub 2018 Mar 27. Semin Immunol. 2018. PMID: 29602515 Review. - Igniting the flame in arthritis: C5aR2 controls endothelial transcytosis of C5a.
Köhl J. Köhl J. Sci Immunol. 2019 May 10;4(35):eaax0352. doi: 10.1126/sciimmunol.aax0352. Sci Immunol. 2019. PMID: 31076526 Review.
Cited by
- Relationship between the complement system and serum lipid profile in patients with rheumatoid arthritis.
Rodríguez-González D, García-González M, Gómez-Bernal F, Quevedo-Abeledo JC, González-Rivero AF, Jiménez-Sosa A, González-López E, Heras-Recuero E, Ocejo-Vinyals JG, González-Gay MÁ, Ferraz-Amaro I. Rodríguez-González D, et al. Front Immunol. 2024 Jul 12;15:1420292. doi: 10.3389/fimmu.2024.1420292. eCollection 2024. Front Immunol. 2024. PMID: 39072319 Free PMC article. - The role of C5a-C5aR1 axis in bone pathophysiology: A mini-review.
Ruocco A, Sirico A, Novelli R, Iannelli S, Van Breda SV, Kyburz D, Hasler P, Aramini A, Amendola PG. Ruocco A, et al. Front Cell Dev Biol. 2022 Aug 8;10:957800. doi: 10.3389/fcell.2022.957800. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36003145 Free PMC article. Review. - Pros and cons of causative association between periodontitis and rheumatoid arthritis.
Koziel J, Potempa J. Koziel J, et al. Periodontol 2000. 2022 Jun;89(1):83-98. doi: 10.1111/prd.12432. Epub 2022 Mar 9. Periodontol 2000. 2022. PMID: 35262966 Free PMC article. Review. - Protective Role of Collectin 11 in a Mouse Model of Rheumatoid Arthritis.
Wang N, Wu W, Qiang C, Ma N, Wu K, Liu D, Wang JX, Yang X, Xue L, Diao TY, Liu JY, Li A, Zhang B, Li ZF, Farrar CA, Banda NK, Bayarri-Olmos R, Garred P, Zhou W, Li K. Wang N, et al. Arthritis Rheumatol. 2021 Aug;73(8):1430-1440. doi: 10.1002/art.41696. Epub 2021 Jul 7. Arthritis Rheumatol. 2021. PMID: 33605085 Free PMC article. - Complement activation on neutrophils initiates endothelial adhesion and extravasation.
Akk A, Springer LE, Yang L, Hamilton-Burdess S, Lambris JD, Yan H, Hu Y, Wu X, Hourcade DE, Miller MJ, Pham CTN. Akk A, et al. Mol Immunol. 2019 Oct;114:629-642. doi: 10.1016/j.molimm.2019.09.011. Epub 2019 Sep 19. Mol Immunol. 2019. PMID: 31542608 Free PMC article.
References
- Firestein, G.S. 1996. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 39:1781–1790. - PubMed
- Choy, E.H., and G.S. Panayi. 2001. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 344:907–916. - PubMed
- Fox, D.A. 1997. The role of T cells in the immunopathogenesis of rheumatoid arthritis: new perspectives. Arthritis Rheum. 40:598–609. - PubMed
- Plater-Zyberk, C., A.J. Hoogewerf, A.E. Proudfoot, C.A. Power, and T.N. Wells. 1997. Effect of a CC chemokine receptor antagonist on collagen induced arthritis in DBA/1 mice. Immunol. Lett. 57:117–120. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical