Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis - PubMed (original) (raw)
. 2002 Dec 2;196(11):1497-506.
doi: 10.1084/jem.20021244.
Eliza Vasile, Dian Feng, Christian Sundberg, Lawrence F Brown, Michael J Detmar, Joel A Lawitts, Laura Benjamin, Xiaolian Tan, Eleanor J Manseau, Ann M Dvorak, Harold F Dvorak
Affiliations
- PMID: 12461084
- PMCID: PMC2194262
- DOI: 10.1084/jem.20021244
Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis
Janice A Nagy et al. J Exp Med. 2002.
Abstract
Vascular permeability factor/vascular endothelial growth factor (VPF/VEGF, VEGF-A) is a multifunctional cytokine with important roles in pathological angiogenesis. Using an adenoviral vector engineered to express murine VEGF-A(164), we previously investigated the steps and mechanisms by which this cytokine induced the formation of new blood vessels in adult immunodeficient mice and demonstrated that the newly formed blood vessels closely resembled those found in VEGF-A-expressing tumors. We now report that, in addition to inducing angiogenesis, VEGF-A(164) also induces a strong lymphangiogenic response. This finding was unanticipated because lymphangiogenesis has been thought to be mediated by other members of the VPF/VEGF family, namely, VEGF-C and VEGF-D. The new "giant" lymphatics generated by VEGF-A(164) were structurally and functionally abnormal: greatly enlarged with incompetent valves, sluggish flow, and delayed lymph clearance. They closely resembled the large lymphatics found in lymphangiomas/lymphatic malformations, perhaps implicating VEGF-A in the pathogenesis of these lesions. Whereas the angiogenic response was maintained only as long as VEGF-A was expressed, giant lymphatics, once formed, became VEGF-A independent and persisted indefinitely, long after VEGF-A expression ceased. These findings raise the possibility that similar, abnormal lymphatics develop in other pathologies in which VEGF-A is overexpressed, e.g., malignant tumors and chronic inflammation.
Figures
Figure 1.
Lymphangiogenic response induced by Ad-VEGF-A164 in nude mouse ears. (a) Normal lymphatic in control ear skin (arrow indicates a valve). (b) Lymphatics at 3 d after Ad-VEGF-A164 are distended from dermal edema but have already enlarged further as the result of endothelial cell division and are transitioning into giant lymphatics. (c–h) Giant lymphatics occupy large portions of the dermis at 7–131 d after Ad-VEGF-A164. Panel c illustrates giant lymphatics that contain colloidal carbon injected as described in Materials and Methods and illustrated macroscopically in Fig. 5. Lymphatic (L) in g contains fibrin clot whereas three unlabeled lymphatics (top right) are filled with colloidal carbon. (i and j) Giant lymphatics wrap around glomeruloid bodies (GB) 13 d after Ad-VEGF-A164. (k) Autoradiograph showing [3H]thymidine incorporation by lymphatic endothelial cells (arrows) at 10 d after Ad-VEGF-A164. L, lymphatics. Giemsa stained 1 μm Epon sections. Bars: a–h, 100 μm; i and j, 50 μm; k, 25 μm.
Figure 2.
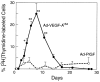
Percent of lymphatic endothelial cells labeled by [3H]thymidine (mean ± std error) at intervals after ear injection with Ad-VEGF-A164 or Ad-PlGF. Values at successive times (based on 3–6 animals per time point) were compared with those at time zero using Dunn's multiple comparisons test. *P < 0.05; **P < 0.01.
Figure 3.
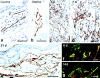
Immunostaining of lymphatics for VEGFR-2 (a–e) and for the hyaluronan receptor LYVE-1 (f and g). Immunoperoxidase staining of control lymphatics (a and b) or giant lymphatics (c–e) at indicated times after Ad-VEGF-A164. Note bridging of lymphatics by VEGFR-2–positive endothelium (d, arrows). L, lymphatics; v, micro blood vessels whose endothelial cells also stain. (f and g) Immunofluorescence staining of lymphatics with anti-LYVE antibody (green) and micro-blood vessels with CD31 (red), 6 and 14 d after Ad-VEGF-A164. Bars: a, b, and e, 10 μm; c and d, 20 μm; f and g, 200 μm.
Figure 4.
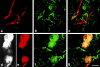
Confocal microscopy of lymphatic and microvascular plexuses in ears of a control mouse (a–c) and a mouse injected 4 d previously with Ad-VEGF-A164 (d–g). FITC-dextran (green) was injected intravenously into the tail vein and TMR-dextran (red) was microinjected into the peripheral ear lymphatics. (a) Normal ear lymphatic plexus delineated by TMR-dextran. (b) Normal ear blood microvasculature delineated by FITC-dextran. (c) Composite retains distinct green and red compartments for the most part, indicating little or no lymphatic uptake of FITC-dextran. (d and e) TMR-dextran, reproduced in both black and white and in red, within a giant lymphatic. Centrally the channel is partially obstructed (presumably by fibrin clot, see text and Figs. 6 and 7), such that the upper and lower portions are connected only through narrow channels that allow tracer flow (best illustrated in d). (f) FITC-dextran fills leaky micro-blood vessels and has entered the lymphatic illustrated in d and e. (g) Merged image. Yellow color indicates FITC-dextran extravasated from leaky blood vessels has been taken up by the giant lymphatic infused with TMR-dextran. Bars, 50 μM.
Figure 5.
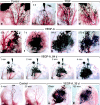
Ear lymphatics after intravital infusion of colloidal carbon in a control mouse and in mice injected at indicated intervals with Ad-PlGF or Ad-VEGF-A164. (a) Control ear. Multiple injection sites (black blotches at top) were required to fill the lymphatic network. Note periodic bulbous swellings that identify valves. (b–d) Lymphatic filling in ears of mice injected at indicated times with Ad-PlGF. Injecting micropipette is shown in place in b. Lymphatics retain bulbous valve markings. (e–h) Pattern of lymphatic filling in ears of mice injected previously, as indicated, with Ad-VEGF-A164. Giant lymphatics are apparent as early as 3 d (e) and persist through day 270. Bulbous valve markings are lost. (i–l) Kinetics of lymphatic filling in ear of a mouse 84 d after injection with Ad-VEGF-A164. Note widespread filling of lymphatic network by 4 min from a single injection site (that with the micropipette in place). An earlier injection site (to left of pipette) failed to engage the terminal lymphatics. (m–p) Clearance of carbon from control ear lymphatics (m and n) is complete by 20 min but at 35 d after Ad-VEGF-A164 ear lymphatics still retain abundant tracer after 150 min (o and p).
Figure 6.
Intraluminal fibrin clot formation and transluminal bridging of giant lymphatics at indicated times after Ad-VEGF-A164 injection. None of the lymphatics illustrated had been injected with carbon or other agents. (a and b) Fibrin clot (*) within giant lymphatics at 10–11 d; note transluminal bridging by lymphatic endothelium. (c–i) Migrating lymphatic endothelial cells formed transluminal bridges across giant lymphatics, dividing their lumens into multiple smaller, endothelium-lined channels. Initially fibrin formed a substrate for endothelial cell migration but was subsequently digested and replaced by collagen. By 22 d (i) bridges had become quite cellular. Note red blood cells within lymphatics (d–f, h). L, giant lymphatics. Giemsa stained 1 μm Epon sections. Bars: a, b, and d–i, 50 μm; c, 25 μm.
Figure 7.
Electron micrographs of giant lymphatics at 21 d (a) and 14 d (b) after Ad-VEGF-A164 injection, illustrating intraluminal fibrin deposits and transluminal bridging by lymphatic endothelial cells. Black arrows indicate intralymphatic fibrin and open arrows the endothelial cell bridging that followed. (c) Intraluminal fibrin is more clearly demonstrated at higher magnification. L, lymphatic lumens. Bars: a and b, 5 μm; c, 1 μm.
Similar articles
- Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine.
Brown LF, Detmar M, Claffey K, Nagy JA, Feng D, Dvorak AM, Dvorak HF. Brown LF, et al. EXS. 1997;79:233-69. doi: 10.1007/978-3-0348-9006-9_10. EXS. 1997. PMID: 9002222 Review. - Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis.
Dvorak HF, Brown LF, Detmar M, Dvorak AM. Dvorak HF, et al. Am J Pathol. 1995 May;146(5):1029-39. Am J Pathol. 1995. PMID: 7538264 Free PMC article. Review. - VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses.
Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, Kholová I, Kauppinen RA, Achen MG, Stacker SA, Alitalo K, Ylä-Herttuala S. Rissanen TT, et al. Circ Res. 2003 May 30;92(10):1098-106. doi: 10.1161/01.RES.0000073584.46059.E3. Epub 2003 Apr 24. Circ Res. 2003. PMID: 12714562 - Complementary and coordinated roles of the VEGFs and angiopoietins during normal and pathologic vascular formation.
Gale NW, Thurston G, Davis S, Wiegand SJ, Holash J, Rudge JS, Yancopoulos GD. Gale NW, et al. Cold Spring Harb Symp Quant Biol. 2002;67:267-73. doi: 10.1101/sqb.2002.67.267. Cold Spring Harb Symp Quant Biol. 2002. PMID: 12858549 No abstract available. - Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor-164 gene delivery.
Sundberg C, Nagy JA, Brown LF, Feng D, Eckelhoefer IA, Manseau EJ, Dvorak AM, Dvorak HF. Sundberg C, et al. Am J Pathol. 2001 Mar;158(3):1145-60. doi: 10.1016/S0002-9440(10)64062-X. Am J Pathol. 2001. PMID: 11238063 Free PMC article.
Cited by
- Lymphangiogenesis and cancer.
Christiansen A, Detmar M. Christiansen A, et al. Genes Cancer. 2011 Dec;2(12):1146-58. doi: 10.1177/1947601911423028. Genes Cancer. 2011. PMID: 22866206 Free PMC article. - Integrins and their extracellular matrix ligands in lymphangiogenesis and lymph node metastasis.
Chen J, Alexander JS, Orr AW. Chen J, et al. Int J Cell Biol. 2012;2012:853703. doi: 10.1155/2012/853703. Epub 2012 Feb 12. Int J Cell Biol. 2012. PMID: 22505936 Free PMC article. - DDA suppresses angiogenesis and tumor growth of colorectal cancer in vivo through decreasing VEGFR2 signaling.
Huang SW, Lien JC, Kuo SC, Huang TF. Huang SW, et al. Oncotarget. 2016 Sep 27;7(39):63124-63137. doi: 10.18632/oncotarget.11152. Oncotarget. 2016. PMID: 27517319 Free PMC article. - Macrophage-mediated lymphangiogenesis: the emerging role of macrophages as lymphatic endothelial progenitors.
Ran S, Montgomery KE. Ran S, et al. Cancers (Basel). 2012 Sep;4(3):618-57. doi: 10.3390/cancers4030618. Epub 2012 Jun 27. Cancers (Basel). 2012. PMID: 22946011 Free PMC article. - Lymphotoxin beta receptor signaling is required for inflammatory lymphangiogenesis in the thyroid.
Furtado GC, Marinkovic T, Martin AP, Garin A, Hoch B, Hubner W, Chen BK, Genden E, Skobe M, Lira SA. Furtado GC, et al. Proc Natl Acad Sci U S A. 2007 Mar 20;104(12):5026-31. doi: 10.1073/pnas.0606697104. Epub 2007 Mar 14. Proc Natl Acad Sci U S A. 2007. PMID: 17360402 Free PMC article.
References
- Hanahan, D. 1997. Signaling vascular morphogenesis and maintenance. Science. 277:48–50. - PubMed
- Folkman, J. 1997. Angiogenesis and angiogenesis inhibition: an overview. EXS. 79:1–8. - PubMed
- Beck, L., Jr., and P.A. D'Amore. 1997. Vascular development: cellular and molecular regulation. FASEB J. 11:365–373. - PubMed
- Gale, N.W., and G.D. Yancopoulos. 1999. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 13:1055–1066. - PubMed
- Brown, L.F., M. Detmar, K. Claffey, J.A. Nagy, D. Feng, A.M. Dvorak, and H.F. Dvorak. 1997. Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. EXS. 79:233–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA 92644/CA/NCI NIH HHS/United States
- HL 59316/HL/NHLBI NIH HHS/United States
- R01 CA050453/CA/NCI NIH HHS/United States
- R01 CA069184/CA/NCI NIH HHS/United States
- P01 CA092644/CA/NCI NIH HHS/United States
- AI 33372/AI/NIAID NIH HHS/United States
- CA 91861/CA/NCI NIH HHS/United States
- CA 69184/CA/NCI NIH HHS/United States
- R01 AI033372/AI/NIAID NIH HHS/United States
- CA 50453/CA/NCI NIH HHS/United States
- R01 CA086410/CA/NCI NIH HHS/United States
- AI 44066/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources