Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells - PubMed (original) (raw)
Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells
Akiyoshi Uemura et al. J Clin Invest. 2002 Dec.
Abstract
Interactions between endothelial cells (ECs) and perivascular mural cells (MCs) via signaling molecules or physical contacts are implicated both in vascular remodeling and maintenance of vascular integrity. However, it remains unclear how MCs regulate the morphogenic activity of ECs to form an organized vascular architecture, comprising distinct artery, vein, and capillary, from a simple mesh-like network. A clear elucidation of this question requires an experimental model system in which ECs are separated from MCs and yet form vascular structures. Here we report that injection of an antagonistic mAb against PDGFR-beta into murine neonates provides such an experimental system in the retina by completely blocking MC recruitment to developing vessels. While a vascular network was formed even in the absence of MCs, it was poorly remodeled and leaky. Using this vascular system ideal for direct assessment of the activities of MC-derived molecules, we show that addition of recombinant modified angiopoietin-1 restored a hierarchical vasculature, and also rescued retinal edema and hemorrhage in the complete absence of MCs. These observations demonstrate the potential of Ang1 as a new therapeutic modality for MC dropout in diseases such as diabetic retinopathies.
Figures
Figure 1
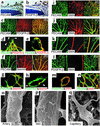
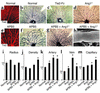
MC recruitment in postnatal development of the retinal vasculature. (a and b) Counterstaining of P5 retina with hematoxylin and PECAM (a) or PDGFR-β (b). In the retina, PDGFR-β is expressed only around developing vessels (arrowheads) arising from the optic nerve (ON). (c–n) Double immunofluorescent staining using PECAM, PDGFR-β, desmin, and SMA. From the beginning of vascular development, PDGFR-β is expressed around the entire retinal vascular network (c), except for sprouting tips (arrowheads in d). PDGFR-β is expressed in MCs located in the outer linings of endothelial surfaces (e). Desmin is expressed in all types of retinal vessels (f), including the growing vascular edges (g), with filamentous patterns in PDGFR-β+ MCs (arrowheads in h). SMA expression over the primary vascular plexus (i and j) is maintained in arterial walls and fades in venous and capillary walls as vascular remodeling proceeds (k). Finally, SMA expression in MCs is confined to arteries (l and m) and newly formed vessels in the advancing regions (n). (o–q) Scanning electron microscopy demonstrating morphological heterogeneity of MCs in accordance with the types of vessels: multiconcentric MCs around arteries (o), single-layer MCs around veins (p), and capillary pericytes with long processes oriented longitudinally along the axis of vessel walls (arrowhead in q).
Figure 2
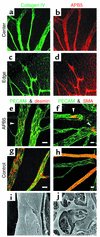
Inhibition of MC recruitment to retinal vessels by postnatal treatment with anti–PDGFR-β Ab. (a–d) Distribution of systemically administered APB5 in the retina. APB5 (rat anti-mouse PDGFR-β mAb) was labeled with anti-rat Ab 3 hours after an intraperitoneal injection into a P4 neonate. Distribution of APB5 (b and d) coincided with the retinal vascular networks, as illustrated by type IV collagen (a and c). (e–h) Double immunofluorescent staining of PECAM and desmin at P5 (e and g), and PECAM and SMA at P8 (f and h). In contrast to the successive MC coverage in the control (g and h), APB5-treated mice failed to recruit MCs in the developing retina, with the exception of the continued presence of a few MCs (arrowheads) only at the roots of trunk vessels arising from the optic disc (e and f). Bars represent 20 μm. (i and j) Scanning electron microscopy depicting denuded endothelial tubes with complete absence of MC coverage in large vessels (i) and capillaries (j) in APB5-treated retina.
Figure 3
Disorganized vascular remodeling in the absence of MCs. (a and b) Double immunostaining with PECAM (brown) and SMA (blue) of P8 retina. In the control (a), advancing vessels had reached the edges of the retina, while in the APB5-treated mice, the peripheral retina remained unvascularized (b). Note the strong SMA expression around arteries in the control retina. (c and d) PECAM staining of P8 retina of control (c) and APB5-treated mice (d). Note the remaining capillary pruning and intense PECAM staining in arteries even in the absence of MCs. (e and f) Morphometric analysis of retinal vasculature at P3, P5, and P8. Black bars, control mice; gray bars, APB5-treated mice. Data (mean ± SD) are expressed as percentages relative to the control retina on P3. *P < 0.05 by Student t test. (g and h) High-magnification view of PECAM staining in P5 retina. The shape of each EC is illustrated by white lines. Increases in the number and size of ECs contributed to vessel enlargement in the absence of MCs. Bars represent 20 μm. (i and j) Detection of dying ECs by TUNEL assay (i) and in vivo propidium iodide labeling (j) in P5 retina. Endothelial apoptosis (arrowheads) was not accelerated even in the absence of MCs. (k–n) Double labeling of BrdU-incorporating cells (green) and PECAM+ ECs (red) in P5 retina. ECs were mitotically active, particularly in the regions of advancing retinal vessels in both APB5-treated mice (m and n) and controls (k and l). A, artery; V, vein.
Figure 4
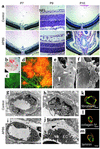
Deteriorated integrity of retinal vessels in the absence of MCs. (a) H&E staining of retinal sections of control (top) and APB5-treated (bottom) mice. APB5 treatment induced progressive exacerbation of retinal edema in the nerve fiber layer (NFL) at around P8 to P9, resulting in retinal collapse and massive hemorrhage at P10. (b) Retinal cup of APB5-treated mouse at P9 depicting widespread hemorrhage and funnel-shaped retinal collapse. (c and d) Visualization of vascular leakage in P8 retina by TRITC-dextran perfusion. Multifocal leakage of systemically perfused dextran demonstrated the increased vascular permeability in the absence of MCs (d). No leakage was found in the control retina (c). (e and f) Scanning electron microscopy demonstrating numerous cleft formations (arrowheads in e) in denuded endothelial sheets and extravasation of blood cells (f). (g–j) Ultrastructural analysis depicting vessel walls with MC coverage in the control retina (g and h) and MC-free endothelial walls in the APB5-treated retina (i and j). In the absence of MCs, extraluminal spaces were enlarged with fluid accumulation. Note the swelling of periendothelial basement membranes with cell debris (arrowhead in j). EC, endothelial cell; MC, mural cell. (k–m) Double immunofluorescent staining of PECAM (green) and fibronectin (k), type IV collagen (l), and laminin (m) (red) in retinal vessels of APB5-treated mice. Major extracellular matrix components of basement membranes were deposited around EC surfaces in the absence of MCs. Bars represent 5 μm.
Figure 5
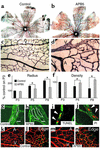
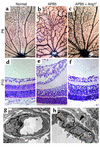
Effects of Ang1 on retinal vascular development. (a–g) P4 retinal vasculature stained with Tie2 (a and e) or with PECAM-1 (b–d, f, and g). Normally, Tie2 is expressed in all retinal vessels (a). Intraocular injections of Tie2-Fc (c) and Ang1* (d) displayed clear contrasts in effects on vascular remodeling, especially in generation of trunk vessels. In the APB5-treated mice, Tie2 is still expressed in all retinal vessels even in the absence of MCs (e). Disorganized vascular architecture induced by systemic APB5 treatment (f) was ameliorated to a great extent by intraocular injections of Ang1* (g). (h) Scanning electron microscopy demonstrating complete absence of MCs in the retina treated with APB5 + Ang1*. (i–m) Quantitative analysis of the radius (i), density (j), arterial diameter (k), venous diameter (l), and capillary diameter (m) of retinal vasculature at P4. Data (mean ± SD) are expressed as percentages relative to the normal retina. *P < 0.05 versus normal (Student t test).
Figure 6
Restoration of hierarchical vascular architecture and vessel integrity by Ang1*. (a–c) PECAM staining of the P8 retina of normal (a), APB5-treated (b), and APB5 + Ang1*–treated (c) mice. Disorganized vascular remodeling induced by APB5 (b) was substantially restored by Ang1*, with clearer distinctions between arteries and veins (c). Note the formation of numerous capillaries and extensive anastomoses with arteries. (d–f) H&E staining of P10 retina of normal (d), APB5-treated (e), and APB5 + Ang1*–treated (f) mice. Ang1* prevented APB5-induced retinal collapse by suppression of edema and hemorrhage. (g and h) Ultrastructural analysis of the vessel walls in the retina treated with APB5 + Ang1*. Despite the absence of MCs, extraluminal fluid accumulation almost completely disappeared (g) and basement membranes were corrected to form a fine layer (arrowhead in h) as seen in the normal vessels. Note the intact integrity of the EC-EC junctions. EC, endothelial cell.
Comment in
- Getting Tie(2)d up in angiogenesis.
Ramsauer M, D'Amore PA. Ramsauer M, et al. J Clin Invest. 2002 Dec;110(11):1615-7. doi: 10.1172/JCI17326. J Clin Invest. 2002. PMID: 12464666 Free PMC article. No abstract available.
Similar articles
- Getting Tie(2)d up in angiogenesis.
Ramsauer M, D'Amore PA. Ramsauer M, et al. J Clin Invest. 2002 Dec;110(11):1615-7. doi: 10.1172/JCI17326. J Clin Invest. 2002. PMID: 12464666 Free PMC article. No abstract available. - Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding EGF-like growth factor.
Iivanainen E, Nelimarkka L, Elenius V, Heikkinen SM, Junttila TT, Sihombing L, Sundvall M, Maatta JA, Laine VJ, Yla-Herttuala S, Higashiyama S, Alitalo K, Elenius K. Iivanainen E, et al. FASEB J. 2003 Sep;17(12):1609-21. doi: 10.1096/fj.02-0939com. FASEB J. 2003. PMID: 12958167 - Angiopoietin 2 expression in the retina: upregulation during physiologic and pathologic neovascularization.
Hackett SF, Ozaki H, Strauss RW, Wahlin K, Suri C, Maisonpierre P, Yancopoulos G, Campochiaro PA. Hackett SF, et al. J Cell Physiol. 2000 Sep;184(3):275-84. doi: 10.1002/1097-4652(200009)184:3<275::AID-JCP1>3.0.CO;2-7. J Cell Physiol. 2000. PMID: 10911358 Review. - Peripheral mural cell recruitment requires cell-autonomous heparan sulfate.
Stenzel D, Nye E, Nisancioglu M, Adams RH, Yamaguchi Y, Gerhardt H. Stenzel D, et al. Blood. 2009 Jul 23;114(4):915-24. doi: 10.1182/blood-2008-10-186239. Epub 2009 Apr 27. Blood. 2009. PMID: 19398718 - Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome.
Stalmans I. Stalmans I. Verh K Acad Geneeskd Belg. 2005;67(4):229-76. Verh K Acad Geneeskd Belg. 2005. PMID: 16334858 Review.
Cited by
- Angiopoietin-1 and Angiopoietin-2 in metabolic disorders: therapeutic strategies to restore the highs and lows of angiogenesis in diabetes.
Isidori AM, Venneri MA, Fiore D. Isidori AM, et al. J Endocrinol Invest. 2016 Nov;39(11):1235-1246. doi: 10.1007/s40618-016-0502-0. Epub 2016 Jun 25. J Endocrinol Invest. 2016. PMID: 27344309 Review. - Neonatal brain hemorrhage (NBH) of prematurity: translational mechanisms of the vascular-neural network.
Lekic T, Klebe D, Poblete R, Krafft PR, Rolland WB, Tang J, Zhang JH. Lekic T, et al. Curr Med Chem. 2015;22(10):1214-38. doi: 10.2174/0929867322666150114152421. Curr Med Chem. 2015. PMID: 25620100 Free PMC article. Review. - Multimodality imaging of abnormal vascular perfusion and morphology in preclinical 9L gliosarcoma model.
Darpolor MM, Molthen RC, Schmainda KM. Darpolor MM, et al. PLoS One. 2011 Jan 31;6(1):e16621. doi: 10.1371/journal.pone.0016621. PLoS One. 2011. PMID: 21305001 Free PMC article. - Endogenous VEGF is required for visual function: evidence for a survival role on müller cells and photoreceptors.
Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, Darland DC, Young MJ, D'Amore PA. Saint-Geniez M, et al. PLoS One. 2008;3(11):e3554. doi: 10.1371/journal.pone.0003554. Epub 2008 Nov 3. PLoS One. 2008. PMID: 18978936 Free PMC article. - Fit for the Eye: Aptamers in Ocular Disorders.
Drolet DW, Green LS, Gold L, Janjic N. Drolet DW, et al. Nucleic Acid Ther. 2016 Jun;26(3):127-46. doi: 10.1089/nat.2015.0573. Epub 2016 Jan 12. Nucleic Acid Ther. 2016. PMID: 26757406 Free PMC article. Review.
References
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. - PubMed
- Yancopoulos GD, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. - PubMed
- Beck L, Jr, D’Amore PA. Vascular development: cellular and molecular regulation. FASEB J. 1997;11:365–373. - PubMed
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. - PubMed
- Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13:1055–1066. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous