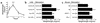T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase - PubMed (original) (raw)
. 2002 Dec;110(11):1739-47.
doi: 10.1172/JCI15695.
Lane L Clarke, Daniel Mamah, Lara R Gawenis, Zheng Zhang, William Ellsworth, David Shalowitz, Navdha Mittal, Petros Efthimiou, Ziad Alnadjim, Steve D Hurst, Eugene B Chang, Terrence A Barrett
Affiliations
- PMID: 12464679
- PMCID: PMC151630
- DOI: 10.1172/JCI15695
T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase
Mark W Musch et al. J Clin Invest. 2002 Dec.
Abstract
Inflammatory bowel disease (IBD) is associated with mucosal T cell activation and diarrhea. We found that T cell activation with anti-CD3 mAb induces profound diarrhea in mice. Diarrhea was quantified by intestinal weight-to-length (wt/l) ratios, mucosal Na(+)/K(+)-ATPase activity was determined and ion transport changes were measured in Ussing chambers. Anti-CD3 mAb increased jejunal wt/l ratios by more than 50% at 3 hours, returning to base line after 6 hours. Fluid accumulation was significantly reduced in TNF receptor-1 (TNFR-1(-/-)), but not IFN-gamma knockout mice. Anti-CD3 mAb decreased mucosal Na(+)/K(+)-ATPase activity, which was blocked by anti-TNF mAb and occurred to a lesser degree in TNFR-1(-/-) mice. Neither alpha nor beta subunits of Na(+)/K(+)-ATPase decreased in abundance at 3 hours. Intestinal tissue from anti-CD3-treated mice exhibited increased permeability to mannitol at 1 hour and decreases in electroneutral Na(+) absorption, Na(+)-dependent glucose absorption, and cAMP-stimulated anion secretion at 3 hours. Furthermore, enteral fluid accumulation was observed in CFTR(-/-) mice, indicating a minor role of active anion secretion. These data suggest that diarrhea in IBD is due to TNF-mediated malabsorption rather than to secretory processes. T cell activation induces luminal fluid accumulation by increasing mucosal permeability and reducing epithelial Na(+)/K(+)-ATPase activity leading to decreased intestinal Na(+) and water absorption.
Figures
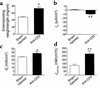
Figure 1
(a) Time course of effect of anti-CD3 mAb on jejunal wt/l ratio. Mice were injected with 0.2 mg anti-CD3 mAb at time 0. Loop wt/l ratios were measured after varying times. Values are means ± SE for six determinations. (b) Effect of anti-TNF and anti–IFN-γ antibodies on jejunal wt/l ratios at 3 hours. Mice were injected simultaneously with 0.2 mg control or anti-CD3 mAb along with neutralizing mAb to TNF or IFN-γ. (c) Control or anti-CD3 mAb was injected in C57BL/6 or in TNFR-1 or IFN-γ knockout mice. Weight-to-length ratios were determined after 3 hours. Values are means ± SE for six determinations in each group. In all cases, *P < 0.05, **P < 0.01 by comparison using ANOVA. In (a), all comparisons were with 0-time control. In (b) and (c), control mAb-treated, anti–CD3-stimulated mice were initially compared with control mAb-treated, unstimulated controls. In (b), results in anti-TNF mAb and anti–IFN-γ–treated, anti–CD3-stimulated mice were compared with control mAb-treated, anti–CD3-stimulated mice. In (c), results in anti–CD3-stimulated TNFR-1–/– and IFN-γ–/– mice were compared with anti–CD3-stimulated B6 mice. The data indicate that TNF but not IFN-γ inhibition prevented anti–CD3-induced diarrhea.
Figure 2
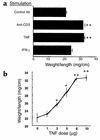
Effect of TNF or IFN-γ on wt/l ratios. (a) Mice were injected with control antibody, anti-CD3 mAb, TNF, or IFN-γ. Jejunal wt/l ratios were measured at 3 hours. Values are means ± SE for six determinations. (b) Dose-dependence of TNF effect. Mice were injected with varying amounts of TNF, and jejunal wt/l ratios were measured at 3 hours. Values are means ± SE for six determinations in each group or each TNF dose. *P < 0.05, **P < 0.01 compared with control antibody in a and 0 TNF in b by ANOVA.
Figure 3
(a–d) Effect of anti-CD3 mAb on Na+/K+-ATPase activity. Mice were injected with control or anti-CD3 mAb and enzyme activity measured in jejunum isolated 3 hours later. Values are means ± SE for four to six determinations in each group. Results in unstimulated mice treated with anti-TNF (a) or anti–IFN-γ (b) mAbs were not different from control mAb-treated, unstimulated mice (data not shown). In (a) and (b), results in control mAb-treated, anti–CD3-stimulated mice were compared with unstimulated mice. Results of anti–cytokine-treated, anti–CD3-stimulated mice were compared with control mAb-treated, anti–CD3-stimulated mice. In (c), data in B6, anti–CD3-stimulated mice were compared with B6, unstimulated mice and results in anti-CD3-stimulated, TNFR-1–/– and IFN-γ–/– mice were compared with B6, anti–CD3-stimulated mice. In (d), results in anti-CD3 mAb-stimulated, TNF-treated and IFN-γ–treated mice were compared with control mAb-treated mice. (e) Effect of anti-CD3 mAb on abundance of Na+/K+-ATPase α and β subunits. Microsomal membranes were isolated and analyzed on SDS-PAGE, and Western blots were performed using mAb to the α and β subunits. *P < 0.05, **P < 0.01 compared with control by ANOVA.
Figure 4
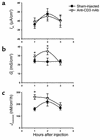
Time course of effect of anti-CD3 mAb on bioelectric parameters and intestinal permeability of murine jejunum. Mice were injected with PBS (Sham-injected) or 0.2 mg anti-CD3 mAb at time 0. Jejunal sections were mounted in Ussing chambers for measurement of _I_sc, _G_t, and _J_mannitol. Values are means ± SE for 15 sections (eight mice) at 1 hour, eight sections (five mice) at 2 hours, and eight sections (four mice) at 3 hours. *P < 0.05, **P < 0.1 vs. sham-injected.
Figure 5
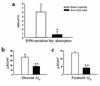
Effect of anti-CD3 mAb on Na+ absorption and anion secretion across murine jejunum. Mice were injected with PBS (Sham-injected) or 0.2 mg anti-CD3 mAb 3 hours before sacrifice. Jejunal sections were mounted in Ussing chambers for measurement of _I_sc and 22Na flux measurements. (a) Mucosal-to-serosal 22Na flux sensitive to 100 μM EIPA (n = 10 sections from six mice). (b) _I_sc response to addition of 10 mM glucose in mucosal bath (15 minutes) to stimulate Na+-coupled glucose transport (n = 12 sections from six mice). (c) _I_sc response to addition of 10 μM forskolin in bathing solutions (10 minutes) to stimulate cAMP-dependent anion secretion (n = 11 sections from six mice). Values are means ± SE. *P < 0.05, **P < 0.1 vs. sham-injected.
Figure 6
Effect of anti-CD3 mAb in CFTR-deficient mice. Mice were injected with PBS (Sham-injected) or 0.2 mg anti-CD3 mAb 3 hours before sacrifice. (a) Effect on jejunal wt/l ratio. Values are means ± SE for three mice in each group. *P < 0.5 vs. sham-injected. (b–d) Effect of anti-CD3 mAb I
SC
, Gt and _J_mannitol of intestine from CFTR-deficient murine jejunum. Values are means ± SE for seven sections (four mice). *P < 0.05, **P < 0.1 vs. sham-injected.
Similar articles
- Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo.
Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Clayburgh DR, et al. J Clin Invest. 2006 Oct;116(10):2682-94. doi: 10.1172/JCI29218. J Clin Invest. 2006. PMID: 17016558 Free PMC article. - Acute inflammation alters bicarbonate transport in mouse ileum.
Zhang H, Ameen N, Melvin JE, Vidyasagar S. Zhang H, et al. J Physiol. 2007 Jun 15;581(Pt 3):1221-33. doi: 10.1113/jphysiol.2007.129262. Epub 2007 Mar 29. J Physiol. 2007. PMID: 17395634 Free PMC article. - Defining the roles of perforin, Fas/FasL, and tumour necrosis factor alpha in T cell induced mucosal damage in the mouse intestine.
Merger M, Viney JL, Borojevic R, Steele-Norwood D, Zhou P, Clark DA, Riddell R, Maric R, Podack ER, Croitoru K. Merger M, et al. Gut. 2002 Aug;51(2):155-63. doi: 10.1136/gut.51.2.155. Gut. 2002. PMID: 12117872 Free PMC article. - Role of epithelial ion transports in inflammatory bowel disease.
Magalhães D, Cabral JM, Soares-da-Silva P, Magro F. Magalhães D, et al. Am J Physiol Gastrointest Liver Physiol. 2016 Apr 1;310(7):G460-76. doi: 10.1152/ajpgi.00369.2015. Epub 2016 Jan 7. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 26744474 Review. - Diarrhea in ulcerative colitis. The role of altered colonic sodium transport.
Greig E, Sandle GI. Greig E, et al. Ann N Y Acad Sci. 2000;915:327-32. doi: 10.1111/j.1749-6632.2000.tb05260.x. Ann N Y Acad Sci. 2000. PMID: 11193595 Review.
Cited by
- Long Non-Coding RNA ANRIL Regulates Inflammatory Factor Expression in Ulcerative Colitis Via the miR-191-5p/SATB1 Axis.
Yu KQ, Li CF, Ye L, Song Y, Wang YH, Lin YR, Liao ST, Mei ZC, Lv L. Yu KQ, et al. Inflammation. 2024 Apr;47(2):513-529. doi: 10.1007/s10753-023-01925-z. Epub 2023 Nov 21. Inflammation. 2024. PMID: 37985573 - Intestinal Immune Imbalance is an Alarm in the Development of IBD.
Hu C, Liao S, Lv L, Li C, Mei Z. Hu C, et al. Mediators Inflamm. 2023 Jul 31;2023:1073984. doi: 10.1155/2023/1073984. eCollection 2023. Mediators Inflamm. 2023. PMID: 37554552 Free PMC article. Review. - Feeding mode influences dynamic gut microbiota signatures and affects susceptibility to anti-CD3 mAb-induced intestinal injury in neonatal mice.
Subramanian S, Geng H, Du C, Chou PM, Bu HF, Wang X, Swaminathan S, Tan SC, Ridlon JM, De Plaen IG, Tan XD. Subramanian S, et al. Am J Physiol Gastrointest Liver Physiol. 2022 Sep 1;323(3):G205-G218. doi: 10.1152/ajpgi.00337.2021. Epub 2022 Jul 12. Am J Physiol Gastrointest Liver Physiol. 2022. PMID: 35819158 Free PMC article. - Intestinal Barrier Dysfunction in Fatty Liver Disease: Roles of Microbiota, Mucosal Immune System, and Bile Acids.
Gupta B, Rai R, Oertel M, Raeman R. Gupta B, et al. Semin Liver Dis. 2022 May;42(2):122-137. doi: 10.1055/s-0042-1748037. Epub 2022 Jun 23. Semin Liver Dis. 2022. PMID: 35738255 Free PMC article. - Tacrolimus-binding protein FKBP8 directs myosin light chain kinase-dependent barrier regulation and is a potential therapeutic target in Crohn's disease.
Zuo L, Kuo WT, Cao F, Chanez-Paredes SD, Zeve D, Mannam P, Jean-François L, Day A, Vallen Graham W, Sweat YY, Shashikanth N, Breault DT, Turner JR. Zuo L, et al. Gut. 2023 May;72(5):870-881. doi: 10.1136/gutjnl-2021-326534. Epub 2022 May 10. Gut. 2023. PMID: 35537812 Free PMC article.
References
- Ciancio MJ, Chang EB. Epithelial secretory response to inflammation. Ann NY Acad Sci. 1992;664:210–221. - PubMed
- Baert FJ, et al. Tumor necrosis factor-alpha antibody (Infliximab) therapy profoundly down-regulates the inflammation in Crohn’s ileocolitis. Gastroenterology. 1999;116:22–28. - PubMed
- Bell SJ, Kamm MA. Antibodies to tumor necrosis factor alpha as treatment for Crohn’s disease. Lancet. 2000;355:858–860. - PubMed
- van Dullemen HM, et al. Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–135. - PubMed
- Casellas F, et al. Intraluminal colonic release of immunoreactive tumour necrosis factor in chronic ulcerative colitis. Clin Sci (Lond) 1994;87:453–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK-54778/DK/NIDDK NIH HHS/United States
- R01 DK054778/DK/NIDDK NIH HHS/United States
- R01 DK048816/DK/NIDDK NIH HHS/United States
- R29 DK048816/DK/NIDDK NIH HHS/United States
- R01 DK038510/DK/NIDDK NIH HHS/United States
- DK-47073/DK/NIDDK NIH HHS/United States
- DK-48816/DK/NIDDK NIH HHS/United States
- DK-38510/DK/NIDDK NIH HHS/United States
- R37 DK047722/DK/NIDDK NIH HHS/United States
- R56 DK048816/DK/NIDDK NIH HHS/United States
- DK-47722/DK/NIDDK NIH HHS/United States
- R01 DK047722/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical