Highly efficient modification of bacterial artificial chromosomes (BACs) using novel shuttle vectors containing the R6Kgamma origin of replication - PubMed (original) (raw)
Highly efficient modification of bacterial artificial chromosomes (BACs) using novel shuttle vectors containing the R6Kgamma origin of replication
Shiaoching Gong et al. Genome Res. 2002 Dec.
Abstract
Bacterial artificial chromosome (BAC) mediated transgenesis has proven to be a highly reliable way to obtain accurate transgene expression for in vivo studies of gene expression and function. A rate-limiting step in use of this technology to characterize large numbers of genes has been the process with which BACs can be modified by homologous recombination in Escherichia coli. We report here a highly efficient method for modifying BACs by using a novel set of shuttle vectors that contain the R6Kgamma origin for DNA replication, the E. coli RecA gene for recombination, and the SacB gene for negative selection. These new vectors greatly increased the ease with which one can clone the shuttle vectors, as well as screen for co-integrated and resolved clones. Furthermore, we simplify the shuttle vector cloning to one step by incorporation of a "built-in" resolution cassette for rapid removal of the unwanted vector sequences. This new system has been used to modify a dozen BACs. It is well suited for efficient production of modified BACs for use in a variety of in vivo studies.
Figures
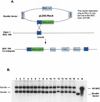
Figure 1
(A) Efficient modification of a Zipro1 BAC by using R6kγ RecA-based shuttle vector. A 500-bp fragment corresponding to the last exon of the Zipro 1 gene (Yang et al. 1997) and an IRESEGFP marker gene were cloned into pLD53-RecA vector. This shuttle vector was then electroporated into Zipro1 containing BAC169 cells (Yang et al. 1997). Co-integrates were formed via homologous recombination and selected by chloramphenicol and ampicillin. (B) Southern blot analysis of the co-integrate BAC clones. Co-integrates BAC DNA were digested with _Spe_I and separated by electrophoresis on a 1% agarose gel. The DNA was transferred to nylon membrane, and the blot was analyzed by using the “A Box” as a probe: co-integrate BAC clones (lanes 1_–_16), wild-type BAC (lane B), and shuttle vector (lane V).
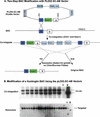
Figure 2
A schematic representation of the two-step BAC modification system with pLD53.SC-AB vector. (A) The pLD53.SC-AB vector is based on the backbone of the plasmid pLD53 (Metcalf et al. 1996). A _Sal_I-_Not_I fragment containing a SacB gene and a RecA gene was subcloned into the pLD53 vector. This shuttle vector carries a R6kγ origin, an ampicillin-resistant gene, and _Asc_I and _Not_I sites as unique cloning sites for subcloning the recombination cassette, which contains a modification (insertion, deletion, or point mutation) flanked by two homology arms of ∼500 bp each. The two BAC modification steps are illustrated: (1) The co-integration of the shuttle vector pLD53.SC-AB into the BAC via homologous recombination through Box A; and (2) the resolution of the co-integrant involves a second homologous recombination event (either through two homology A or two homology B) to eliminate the pLD53 vector and other unnecessary sequences from the co-integrate. The resolved BACs were selected by growth on sucrose owing to the loss of SacB gene. (B) Southern blot analysis of a Huntington BAC clone modified by two-step BAC modification method. Co-integrates and resolved BAC candidates were first screened by PCR (data not shown). Positive clones were selected, and their DNA was digested with _Eco_RI, separated by electrophoresis on a 1% agarose gel, and transferred to nylon membrane. The blots were analyzed using the “A Box” as probes. Controls used were wild-type BAC DNA (B) and shuttle vector (V).
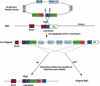
Figure 3
A schematic representation of the two-step BAC modification system with pLD53.SC1 vector. This vector is based on the backbone of the plasmid pLD53 (Metcalf et al. 1996). A _Sal_I-_Not_1 fragment containing a SacB gene, a RecA gene, two IRESEGFP genes, and multiple cloning sites was subcloned into a mutated pLD53. This shuttle vector carries a R6kγ origin, an ampicillin-resistant gene, and _Asc_I and _Sma_I as unique cloning sites for subcloning of Box A. The two BAC modification steps are illustrated: (1) The co-integration of the shuttle vector pLD53.SC1 into the BAC via homologous recombination through Box A, and (2) the resolution of the co-integrant involves a second homologous recombination event (either through two homology A or two IRESEGFP) to eliminate the pLD53 vector and other unnecessary sequences from the co-integrate. The resolved BACs are selected by growth on sucrose owing to the loss of SacB gene.
Figure 4
Southern blot analysis of clones modified by the pLD53.SC1 shuttle vector with the built-in resolution strategy. Co-integrates and resolved BAC DNA were digested with _Spe_I, separated by electrophoresis on a 1% agarose gel, and transferred to nylon membrane. The blots were analyzed using the “A Box” as probes. Controls used were wild-type BAC DNA (lane B) and shuttle vector (lane V).
Similar articles
- Rapid bacterial artificial chromosome modification for large-scale mouse transgenesis.
Gong S, Kus L, Heintz N. Gong S, et al. Nat Protoc. 2010 Sep;5(10):1678-96. doi: 10.1038/nprot.2010.131. Epub 2010 Sep 30. Nat Protoc. 2010. PMID: 20885380 Free PMC article. - Two-Step Bacterial Artificial Chromosome (BAC) Engineering: Preparation of Shuttle Vector DNA.
Heintz N, Gong S. Heintz N, et al. Cold Spring Harb Protoc. 2020 Apr 1;2020(4):098038. doi: 10.1101/pdb.prot098038. Cold Spring Harb Protoc. 2020. PMID: 32238589 - One-Step Bacterial Artificial Chromosome (BAC) Modification: Transfer of the Reporter Vector into BAC/RecA Cells and Selection of Co-Integrates.
Heintz N, Gong S. Heintz N, et al. Cold Spring Harb Protoc. 2020 Jul 1;2020(7):098137. doi: 10.1101/pdb.prot098137. Cold Spring Harb Protoc. 2020. PMID: 32611780 - An overview on the generation of BAC transgenic mice for neuroscience research.
Yang XW, Gong S. Yang XW, et al. Curr Protoc Neurosci. 2005 May;Chapter 5:Unit 5.20. doi: 10.1002/0471142301.ns0520s31. Curr Protoc Neurosci. 2005. PMID: 18428622 Review. - Bacterial artificial chromosome mutagenesis using recombineering.
Narayanan K, Chen Q. Narayanan K, et al. J Biomed Biotechnol. 2011;2011:971296. doi: 10.1155/2011/971296. Epub 2010 Dec 9. J Biomed Biotechnol. 2011. PMID: 21197472 Free PMC article. Review.
Cited by
- Electrophysiological properties of genetically identified subtypes of layer 5 neocortical pyramidal neurons: Ca²⁺ dependence and differential modulation by norepinephrine.
Guan D, Armstrong WE, Foehring RC. Guan D, et al. J Neurophysiol. 2015 Apr 1;113(7):2014-32. doi: 10.1152/jn.00524.2014. Epub 2015 Jan 7. J Neurophysiol. 2015. PMID: 25568159 Free PMC article. - Expression of human E46K-mutated α-synuclein in BAC-transgenic rats replicates early-stage Parkinson's disease features and enhances vulnerability to mitochondrial impairment.
Cannon JR, Geghman KD, Tapias V, Sew T, Dail MK, Li C, Greenamyre JT. Cannon JR, et al. Exp Neurol. 2013 Feb;240:44-56. doi: 10.1016/j.expneurol.2012.11.007. Epub 2012 Nov 12. Exp Neurol. 2013. PMID: 23153578 Free PMC article. - Identification of the human herpesvirus 6A gQ1 domain essential for its functional conformation.
Maeki T, Hayashi M, Kawabata A, Tang H, Yamanishi K, Mori Y. Maeki T, et al. J Virol. 2013 Jun;87(12):7054-63. doi: 10.1128/JVI.00611-13. Epub 2013 Apr 17. J Virol. 2013. PMID: 23596294 Free PMC article. - Use of laser-capture microdissection for the identification of marker genes for the ventromedial hypothalamic nucleus.
Segal JP, Stallings NR, Lee CE, Zhao L, Socci N, Viale A, Harris TM, Soares MB, Childs G, Elmquist JK, Parker KL, Friedman JM. Segal JP, et al. J Neurosci. 2005 Apr 20;25(16):4181-8. doi: 10.1523/JNEUROSCI.0158-05.2005. J Neurosci. 2005. PMID: 15843621 Free PMC article. - Molecular neuroanatomy's "Three Gs": a primer.
Dymecki SM, Kim JC. Dymecki SM, et al. Neuron. 2007 Apr 5;54(1):17-34. doi: 10.1016/j.neuron.2007.03.009. Neuron. 2007. PMID: 17408575 Free PMC article. Review.
References
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. - PubMed
- Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. - PubMed
- Carvajal JJ, Cox D, Summerbell D, Rigby PW. A BAC transgenic analysis of the Mrf4/Myf5 locus reveals interdigitated elements that control activation and maintenance of gene expression during muscle development. Development. 2001;128:1857–1868. - PubMed
- DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. - PubMed
- Filutowicz M, Rakowski SA. Regulatory implications of protein assemblies at the γ origin of plasmid R6K: A review. Gene. 1998;223:195–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources