Butyrate induced Caco-2 cell apoptosis is mediated via the mitochondrial pathway - PubMed (original) (raw)
Butyrate induced Caco-2 cell apoptosis is mediated via the mitochondrial pathway
F M Ruemmele et al. Gut. 2003 Jan.
Abstract
Background: During the process of tumorigenesis most colon cancer cells acquire resistance to apoptosis. The short chain fatty acid butyrate is well established as an antitumour agent which selectively induces apoptosis in colon cancer cells but not in normal intestinal epithelial cells.
Aims: To analyse the signalling pathway of butyrate induced apoptosis.
Methods: Using Caco-2 cells we focused on the bcl family of proteins, mitochondrial pathway, and caspase signalling cascade involved in butyrate induced apoptosis. Techniques employed included western blots, immunofluorescence, as well as experiments with peptide inhibitors of specific caspases.
Results: Butyrate induced a clear shift of the mitochondrial bcl rheostat towards a proapoptotic constellation, as demonstrated by upregulation of proapoptotic bak accompanied by reduced antiapoptotic bcl-x(L) levels. This was associated with translocation of cytochrome-c from the mitochondria to the cytosol, resulting in activation of the caspase cascade via caspase-9. Key executioner enzymes were caspases-3 and -1. No effect of butyrate on regulatory proteins of the inhibitor of apoptosis family was observed.
Conclusions: Butyrate induced Caco-2 cell apoptosis via the mitochondrial pathway. Upregulation of bak and translocation of cytochrome-c were upstream of the caspase cascade. Subsequently, this cascade was activated via the formation of an apoptosome.
Figures
Figure 1
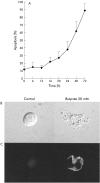
Butyrate induced apoptosis in Caco-2 cells. (A) Kinetic analysis revealed that induction of apoptosis occurred after a lag interval of approximately 16 hours after butyrate 20 mM. (B) Confocal microscopy of untreated and butyrate treated Caco-2 cells. The normal ovaloid homogenous surface is totally altered by membrane blebbing and the formation and segregation of apoptotic bodies in butyrate treated Caco-2 cells. (C) In butyrate stimulated cells, expression of phosphatidyl serine (PS) occurred on the outer leaflet of the plasma membrane, an early sign of loss of normal membrane asymmetry. PS expression was visualised by FITC labelled annexin-V, which binds to PS. No PS expression was observed in control cells.
Figure 2
Activation of the caspase signalling cascade in butyrate stimulated Caco-2 cells. (A) Use of the broad range caspase inhibitor zVAD-fmk confirmed that butyrate induced apoptosis required a functional caspase cascade. Experiments with highly specific peptide inhibitors directed against caspases-3 (zDEVD-fmk), -8 (IETD-fmk), and -9 (LEHD-fmk) revealed that inhibition of caspase-3 as well as caspase-9 strongly reduced butyrate induced apoptosis, to the point of completely blocking it at certain concentrations. In contrast, inhibition of caspase-8 had no effect. (B) Western blot experiments confirmed the role of the caspase signalling cascade in butyrate induced Caco-2 cell apoptosis. Activation of caspase-9 was suggested by a dose dependent decrease of the band of the inactive proenzyme. For caspases-3 and -1, the active cleaved enzyme was observed in response to butyrate. No effect on caspase-8 was observed. The positive control of caspase-8 activation consisted of TRAIL induced apoptotic Caco-2 cells. One of three representative experiments is shown for each blot.
Figure 3
No effect of butyrate on the antiapoptotic proteins of the inhibitor of apoptosis (IAP) family was observed. Baseline levels of X linked inhibitor of apoptosis (XIAP), cIAP1, and cIAP 2 were high in Caco-2 cells; however, they remained unchanged after stimulation with butyrate. One of three representative experiments is shown for each blot.
Figure 4
Translocation of cytochrome-c from the mitochondrial to the cytosolic compartment in butyrate treated Caco-2 cells. (A) After ultracentrifugation to separate the mitochondrial from the cytoplasmic compartments, a shift of cytochrome-c was observed by western blot in response to butyrate. In contrast, a clear and strong signal for apoptosis inducing factor (AIF) in western blots was only observed in the mitochondrial compartment, without any shift of AIF to the cytosolic compartment, even at high butyrate concentrations. (B) In addition, immunofluorescence analysis confirmed that the perinuclear mitochondrial staining for cytochrome-c in unstimulated cells changed to a diffuse pattern of cytosolic staining in cells after stimulation with butyrate. (C) Inhibition of the caspase cascade with the peptide inhibitor zVAD-fmk (200 μM) did not alter the translocation of cytochrome-c, indicating that this event was independent and upstream of the caspase cascade. One of three representative experiments is shown for each blot.
Figure 5
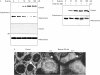
Effect of butyrate on the mitochondrial bcl rheostat. Western blot experiments of whole cell lysates showed that unstimulated Caco-2 cells expressed high levels of antiapoptotic bcl-xL and lower bcl-2, as well as high levels of the proapoptotics bid and bax. Mitochondrial expression of bak, which under unstimulated conditions was rather low, was markedly enhanced after stimulation with butyrate. No effect was seen on bid or bax levels. On the other hand, bcl-xL expression was markedly decreased by butyrate, even at concentrations as low as 1 mM. One of three representative experiments is shown for each blot.
Figure 6
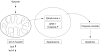
Proposed signalling pathway for butyrate induced Caco-2 cell apoptosis. After stimulation with butyrate the mitochondrial bcl rheostat is shifted towards a proapoptotic constellation, with markedly increased mitochondrial bak levels and decreased bcl-xL levels. This shift allows the translocation of cytochrome-c from the mitochondria to the cytosol, allowing the formation of an apoptosome. Thereafter, activation of the caspase cascade via caspase-9 ensues, leading to apoptotic cell death.
References
- Moss SF, Scholes JV, Holt PR. Abnormalities of epithelial apoptosis in multistep colorectal neoplasia demonstrated by terminal deoxyuridine nick end labeling. Dig Dis Sci 1996;41:2238–47. - PubMed
- Young GP, Gibson PR. Butyrate and the human cancer cell. In: Cumming JJ, Rombeau JL, Sakata T, eds. Physiologic and clinical aspects of short-chain fatty acids. Cambridge: Cambridge University Press, 1995:319–36.
- Hague A, Manning AM, Hanlon KA, et al. Sodium butyrate induces apoptosis in human colonic tumor cell lines in a p53-independent pathway: implication for the possible role of dietary fibers in the prevention of large bowel cancer. Int J Cancer 1993;55:498–505. - PubMed
- Avivi-Green C, Polak-Charcon S, Madar Z, et al. Apoptosis cascade proteins are regulated in vivo by high intracolonic butyrate concentration: correlation with colon cancer inhibition. Oncol Res 2000;12:83–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials